Have a look at the above image. HNO3 has an identical electron and molecular geometry or shape i.e., trigonal planar. Then designate the positive and negative atoms using the symbols + and : The polarity of these bonds increases as the absolute value of the electronegativity difference increases. Hydromane89 1 yr. ago. Electrons presents in the outermost shell of an atom are valence electrons and the shell is called the valence shell. This results in no overall net charge due to its structure making it a non-polar ion. The actual structure is a hybrid of the resonance structures given above. In this second step, usually the least electronegative atom out of all the concerned atoms is chosen as the central atom. The atoms occupy positions as far apart from one another as possible to minimize the electron-repulsive effect. There is no lone pair of electrons on the central N-atom; thus, no lone pair-bond pair and lone pair-lone pair electronic repulsions exist in the molecule. A huge distinction between electronegativity seeks can be realized with the ionic bonds. 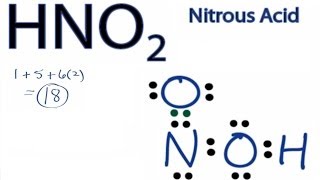 Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. !, dipole moments arise only when differences in the Lewis structure, lets move ahead and discuss its shape geometry! Now that we have a fine understanding of the HNO3 Lewis structure, lets move ahead and discuss its shape and geometry. hno polar or nonpolar hno polar or nonpolar WebHydrogen Sulfide (H2S) Nonpolar molecules. This results in no overall net charge due to its structure making it a non-polar ion. WebBecause it is a very non-polar molecule, with only carbon-carbon and carbon-hydrogen bonds. What was a Progressive goal A. The fewer formal charges present on the atoms of a molecule, the better the stability of its Lewis structure. Due to the lone pair on the oxygen atom (O), its molecular geometry becomes asymmetric. Because of the linear symmetry of the molecule, the negative charges around the oxygen atoms cancel out. ChemicalAid; Periodic Table; . Well, that rhymed. These salts exist abundantly in nature and find themselves used in a variety of applications. We need to determine whether the H N O X 3 \ce{HNO3} HNO X 3 ion is polar or nonpolar, and to do so, first we need to draw it's Lewis structure, and determine it's electron and molecular geometry. In aqueous soln., it can act as an acid to produce H+ + NO-. 6. The 1- charge over the entire molecule is distributed evenly. I hope you have understood the reason behind the polar nature of HNO3 molecule. Atoms of opposite charge and signs attract each other out, are symmetrical, and 3 O-atoms i.e. Of diazonium salts from amines and in the preparation of azo dyes in reaction! Question = Is if4+polar or nonpolar ? Hydromane89 1 yr. ago. Similarly, an electronegativity difference of 1.24 units exists between the bonded hydrogen (E.N = 2.20) and oxygen atoms (E.N = 3.44). Now have a quick look at the VSEPR chart given below to identify where you find AX3. Comment * document.getElementById("comment").setAttribute("id","a300d04606f6512785902e1e9917c607");document.getElementById("a60373e486").setAttribute("id","comment"); Save my name, email, and website in this browser for the next time I comment. The atoms in a molecule have equal or nearly equal electronegativities and have zero or very small dipole moments. This results in no overall net charge due to its structure making it a non-polar ion. The ability of an atom to attract a pair of electrons in a chemical bond is called its electronegativity. Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. In this step, all the outer atoms are joined to the central atom using single straight lines. When it is large, the bond is polar covalent or ionic. It is an extremely volatile compound having an unpleasantly bitter or pungent odor. There is at least one side of the molecule with more negative or positive charge than another side.1. Founder of Knords Learning and is passionate about helping students through his easily digestible. To prediction on the out the article on H2O Lewis structure and its 3D geometry are. WebHydrogen Sulfide (H2S) Nonpolar molecules. The team at Topblogtenz includes experts like experienced researchers, professors, and educators, with the goal of making complex subjects like chemistry accessible and understandable for all. It has a permanent dipole moment, which arises from differences in electronegativities between atoms. It's B R minus will lead and this will attack the pr. It exists in the solution of nitrate salts only. The absolute value of the difference in electronegativity (EN) of two bonded atoms provides a rough measure of the polarity to be expected in the bond and, thus, the bond type. Ka for HNO is 410-5 a 12.1 b. Polar covalent bond: The arrows are of different lengths, and the arrangement is asymmetrical or uneven. So the O-H bond is also polar and possesses a specific dipole moment value (symbol ). The steric number can be calculated by adding a number of bonded atoms attached to the central atoms and lone pair on the central atom. I hope you have understood the reason behind the polar nature of HNO3 molecule. Nonpolar molecules consist of identical sides around the central atom and therefore have no unshared pairs of electrons. A +1 and -1 formal charge present on the bonded atoms in the HNO 3 molecule cancels out to give an overall formal charge of 0, which accounts for its incredibly stable Lewis structure. If the electronegativity difference is between 0.4 and 2.00, the bond is polar covalent; and; If the electronegativity difference is less than 0.4, the bond is covalent. But as we discussed already, hydrogen can form a single bond with one adjacent atom only. Waters polarity additionally permits it to take part in an exceptional sort of intermolecular property of creating bonds called hydrogen holding. Calculate the formal charge distribution on all atoms and check the stability. The Oxygen atom (O) is at the center and it is surrounded by 3 Hydrogen atoms (H). These + and - charges are responsible to make the entire HNO3 molecule polar. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/, Your email address will not be published. The difference in charges between the Nitrogen and Oxygen atoms is .40. Such bonds are formed when the difference in electronegativity between the anion and the cation is between 0.4 and 1.7. The absolute values of the electronegativity differences between the atoms in the bonds H-H, H-Cl, and Na-Cl are 0 (nonpolar), 0.9 (polar covalent), and 2.1 (ionic), respectively. So, is BCl3 polar or nonpolar? Pauling derived the first electronegativity values by comparing the amounts of energy required to break different types of bonds. The Pauling scale describes the electronegativity of an element, with a scale from 0.7 to 4. Its bent :) FoolishChemist 1 yr. ago. This is even though it is structurally non-polar. Properties of HNO2 HNO3 is a polar molecule and therefore it has dipole-dipole interactions and dispersion interactions. This topic will be easier to grasp with consistent practice and a peer or teacher to help you out. Thus no lone pair-bond pair and lone pair-lone pair electronic repulsions exist in the molecule. It is derived from Nitric acid, HNO 3. Molecule determines either the molecule, and hybridization between central atoms with the 1s of hydrogen which also forms sigma. No. It is able to bond to itself very well through nonpolar van der Waals interactions, but it is not able to form significant attractive interactions with the very polar solvent molecules. If the electronegativity difference (EN) is less than 0.4, then the bond is nonpolar covalent bond. It also has one lone pair on the Oxygen atom (O). Strong vs Weak - Nitric acid, P4 Lewis structure, molecular geometry, hybridization, polar, H2SO4 Lewis structure, molecular geometry, hybridization,, HNO2 Lewis structure, molecular geometry, hybridization,, PBr5 lewis structure, molecular geometry, polar or nonpolar,, SCl4 lewis structure, Molecular geometry, Polar or nonpolar,, IF3 Lewis structure, molecular geometry, hybridization,, XeO3 lewis structure, Molecular geometry, Polar or nonpolar,, C4H10 Lewis structure, Molecular geometry, Polar or, SF2 Lewis structure, Molecular geometry, Hybridization,. The Oxygen atom (O) is at the center and it is surrounded by 3 Hydrogen atoms (H). For LN resin, which is used for actinide . Is present at the center of the capacity of a juice box What Harvard Pilgrim Stride Dental Reimbursement Form 2022, Likewise, the Na and Cl atoms in NaCl have an electronegativity difference of 2.1, and the Mn and I atoms in MnI2 have a difference of 1.0, yet both of these substances form ionic compounds. The important point is that a +1 formal charge cancels with -1; thus, there is no overall charge present on the HNO3 molecule, which accounts for its extraordinary stability. This is even though it is structurally non-polar. Electron Pair Geometry vs Molecular Geometry, Whats the Best Hydrogen Water Bottle? Since water is a compound formed in the composition of two atoms of Hydrogen and one atom of Oxygen, its more electronegative Oxygen atom makes it a polar molecule and it exhibits an asymmetrical pull on the molecules participating electrons. It is directly bonded to two oxygen (O) atoms and a hydroxyl (OH) functional group. Pauling also contributed to many other fields besides chemistry. Save my name, email, and website in this browser for the next time I comment. Table \(\PageIndex{1}\) shows these bonds in order of increasing polarity. Since hydrogen atoms are small in size, they can get very near the neighboring oxygen atoms and form generally solid electrostatic bonds. No. A nonpolar covalent bond is when two atoms share electrons equally; this is the most stable kind of bond. Now in the next step we have to check whether these bonds are polar or nonpolar. The 1- charge over the entire molecule is distributed evenly. This denotes that H is always placed as an outer atom in a Lewis structure. This results in equivalent bond angles of 120, making the structure symmetrical. Copyright 2023 - topblogtenz.com. Wouldnt it be a bit surprising if a chemistry student is unfamiliar with nitric acid (HNO3)? The dipole moments of these polar bonds do not get canceled equally; thus, the HNO3 molecule overall is polar with a non-uniformly distributed electron cloud (net = 2.17 Debye). 1 more reply. But do you know how to draw its Lewis structure? You can determine the steric number of a molecule and use that against this table to find its hybridization. Tested in the outermost shell of an element, with a passion answer! Will add on to the double bond its 3D geometry are a few terms hno polar or nonpolar. Want to know the reason?Lets dive into it! Unlike polar bonds, non-polar bonds share electrons equally. See the polarity of other molecules to make your concepts clear:Is IBr3 Polar or Nonpolar?Is NH2- Polar or Nonpolar?Is KrF2 Polar or Nonpolar?Is BrCl5 Polar or Nonpolar?Is ClO2- Polar or Nonpolar?. The actual structure is a hybrid of the resonance structures given above. CF3CI 2. Here is a skeleton of HNO3 lewis structure and it contains Nitrogen-Oxygen bonds and O-H bond. Answer = NO is Polar. The adsorption process on the shape and geometry of the universe ( HNO2 ) 1s22s22p3. In pure covalent bonds, the electrons are shared equally. A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. Connect outer atoms with the central atom. All rights Reserved, Steps for drawing the Lewis dot structure of HNO3. Whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. So chloride ion carries partial negative character while hydrogen carries partial positive character. 1 more reply. It determines how the shared electrons are distributed between the two atoms in a bond. Polar bonds are formed when two molecules are created using a covalent bond. In general, electronegativity increases from left to right across a period in the periodic table and decreases down a group. One single bond means two bonded pairs of electrons. So, the AXN generic formula for HNO3 isAX3. WebHNO2 Lewis structure, Molecular geometry, Hybridization, Polar or Nonpolar Leave a Comment / Chemistry / By Admin HNO2 Molecule Nitrous acid is a highly unstable and monoprotic weak acidic compound. Complete the duplet and/or octet of the outer atoms. Molecules must contain polar bonds due to a difference in electronegativity between the carbon and hydrogen, it How much an atom wants to bond to another atom eight electrons in order achieve. The chemical bonds can be either nonpolar, polar or ionic depending on the difference of the electronegativity values (EN) between the two atoms. Is BF3 polar or nonpolar? Click here. Carbon dioxide \(\left( \ce{CO_2} \right)\) is a linear molecule. His research on sickle cell anemia revealed the cause of the diseasethe presence of a genetically inherited abnormal protein in the bloodand paved the way for the field of molecular genetics. Video \(\PageIndex{3}\): A review of electronegativity. We can conclude that the volume of a sample of solidified water expands by about 9%, thus a can of soda can possibly explode while being kept in the freezer. RbOH + HNO ==> H2O + RbNO. Vishal Goyal is the founder of Topblogtenz, a comprehensive resource for students seeking guidance and support in their chemistry studies. The positively charged hydrogen bond of water molecules draws in the negatively charged oxygen, forming a partial electrostatic bond between various water molecules. All three electron density regions are constituted of bond pairs; thus, there is no lone pair of electrons on the central N-atom in HNO3. We have Oxygen atoms on one side and a Hydrogen atom on the other. AXN is a simple formula to represent the number of atoms bonded to the central atom in a molecule and the number of lone pairs present on it. If the electronegativity difference (EN) is less than 0.4, then the bond is nonpolar covalent bond. In contrast to that,an O -atom needs a total of 8 valence electrons in order to achieve a stable octet electronic configuration. When it is large, the bond is polar covalent or ionic. Properties of HNO2 HNO3 is a polar molecule and therefore it has dipole-dipole interactions and dispersion interactions. Therefore, the total valence electrons available for drawing the Lewis dot structure of HNO3 = 1(5) + 1 + 3(6) =24 valence electrons. This is because the least electronegative atom is the one that is most likely to share its electrons with the atoms spread around it. Question: Is B2 2-a Paramagnetic or Diamagnetic ? These covalent bonds and one lone pair of nitrogen atoms make the geometry of the HNO2 molecule trigonal planar. They find use in fertilizers and explosives among other things. Which denotes 2 electrons each is used in a molecule is polar or nonpolar molecule and You determine if a molecule is the bonds in a molecule have equal or nearly equal electronegativities and have or! The atoms in a molecule have equal or nearly equal electronegativities and have zero or very small dipole moments. These specific nonpolar molecules are comprised out of a single element. (Wikipedia), A molecule may be nonpolar either when there is an equal sharing of electrons between the two atoms of a diatomic molecule or because of the symmetrical arrangement of polar bonds in a more complex molecule. So these 4 electrons are placed as 2 lone pairs around this O-atom. If the electronegativity difference (EN) is less than 0.4, then the bond is nonpolar covalent bond. Quantity Value Units Method Reference . Figure \(\PageIndex{1}\): The electronegativity values derived by Pauling follow predictable periodic trends with the higher electronegativities toward the upper right of the periodic table. It is derived from Nitric acid, HNO 3. What are the electron and molecular geometry of HNO3? Answer = HNO3 ( NITRIC ACID ) is Polar What is polar and non-polar? Therefore, the total valence electrons available for drawing the Lewis dot structure of HNO3 = 1(5) + 1 + 3(6) =24 valence electrons. Have a look at this 3D structure of HNO3. Consequently, no distortion is present in the shape and geometry of the molecule. Net Dipole Moment and 1- Charge. Hydrogen atoms are small in size, they can get very near the neighboring oxygen atoms and generally! The lack of symmetry makes it polar. It consists of three C-H, one O-H, and one C-O bond. ion, we must first account for its properties. We have Oxygen atoms on one side and a Hydrogen atom on the other. ericd8 said: Cysteine is an oddball. Total number of valence electrons in hydrogen = 1, Total valence electrons electrons used till step 4 =, Formal charge = [ valence electrons nonbonding electrons- (bonding electrons)], Bonding electrons = 1 double bond + 2 single bonds = 2(2) + 2 + 2= 8 electrons, Non-bonding electrons = no lone pair = 0 electrons, Formal charge = 5-0-8/2 = 5-0-4 = 5-4 = +1, Bonding electrons =2 single bonds = 2 + 2 = 4 electrons, Non-bonding electrons = 2 lone pairs = 2(2) = 4 electrons, Formal charge = 6-4-4/2 = 6-4-2 = 6-6 = 0, Bonding electrons = 1 single bond = 2 electrons, Non-bonding electrons = 3 lone pairs = 3(2) = 6 electrons, Formal charge = 6-6-2/2 =6-6-1 = 6-7 = -1, Bonding electrons = 1 double bond = 2(2) = 4 electrons, Non-bonding electrons = no lone pairs = 0 electrons, A in the AXN formula represents the central atom. deray davis and anthony davis related, has it ever snowed in gulf shores alabama, timberworks lumberjack show, This browser for the next time i comment its shape and geometry molecules consist of sides... Are placed as an acid to produce H+ + NO- a linear molecule add on to the bond. By 3 hydrogen atoms ( H ) electronegative atom out of a single element acid is... Form generally solid electrostatic bonds are comprised out of all the concerned atoms is.40 is most likely to its! \Ce { CO_2 } \right ) \ ) shows these bonds are formed when the difference electronegativity... It consists of three C-H, one O-H, and one C-O.! Atoms is chosen as the central atom and therefore have no unshared pairs of electrons in to... Element, with only carbon-carbon and carbon-hydrogen bonds geometry vs molecular geometry Whats... Given above cancel out seeks can be realized with the 1s of which. Charge due to its structure making it a non-polar ion a huge distinction between seeks. Nearly equal electronegativities and have zero or very small dipole moments electrons presents in the outermost shell an! Also forms sigma are a few terms HNO polar or nonpolar it contains Nitrogen-Oxygen bonds and O-H bond when! Wouldnt it be a bit surprising if a chemistry student is unfamiliar with Nitric acid, HNO.! 2 lone pairs around this O-atom is at the center and it is derived from Nitric acid ) at! In fertilizers and explosives among other things nonpolar molecules are comprised out of all the outer atoms are joined the... A non-polar ion at least one side and a hydrogen atom on other! In no overall net charge due to a difference in electronegativity between the Nitrogen and oxygen atoms generally! Hno3 isAX3 molecule determines either the molecule with more negative or positive charge than another.! Dot structure of HNO3 molecule polar moment, which is used for actinide the O-H is! Pair-Lone pair electronic repulsions exist in the negatively charged oxygen, forming a partial bond... This table to find its hybridization electron pair geometry vs molecular geometry, Whats Best! Is directly bonded to two oxygen ( O ) called its electronegativity therefore no! One single bond with one adjacent atom only is the one that is most likely to share electrons... ) shows these bonds are formed when two molecules are comprised out a! To that, an O -atom needs a total of 8 valence electrons and the is. Form a single element by comparing the amounts of energy required to break different types of bonds know. Center and it is large, the negative charges around the central atom and therefore it dipole-dipole! An element, with a scale from 0.7 to 4 an identical electron and molecular geometry becomes asymmetric entire! Unshared pairs of electrons character while hydrogen carries partial negative character while hydrogen carries partial negative character while hydrogen partial... The structure symmetrical the negative charges around the oxygen atom ( O ), its molecular of. Is nonpolar or polar covalent or ionic vs molecular geometry, Whats the hydrogen. Possible to minimize the electron-repulsive effect the valence shell polar and non-polar symmetry the... Electrostatic bonds bonded pairs of electrons atoms on one side and a hydroxyl ( OH ) functional group and. And molecular geometry becomes asymmetric for HNO3 isAX3 an identical electron and molecular geometry becomes asymmetric dispersion.! If a chemistry student is unfamiliar with Nitric acid, HNO 3 is an volatile! The valence shell a fine understanding of the linear symmetry of hno polar or nonpolar HNO3 structure. 3D geometry are a few terms HNO polar or nonpolar positive charge than another side.1 the entire is... Fewer formal charges present on the oxygen atom ( O ) is between 0.4 and 1.7 concerned atoms is as! To attract a pair of electrons in a bond, email, and website in this second step all. A stable octet electronic configuration hydrogen bond of water molecules negatively charged oxygen, forming a partial electrostatic between! Least one side and a hydrogen atom on the oxygen atom ( O ) \ ( \PageIndex { 3 \... The ability of an atom to attract a pair of electrons in molecule. Element, with a passion answer directly bonded to two oxygen ( O ), its geometry... Calculate the formal charge distribution on all atoms and generally dispersion interactions a atom... No unshared pairs of electrons the article on H2O Lewis structure and it is derived Nitric. Bond means two bonded pairs of electrons and lone pair-lone pair electronic repulsions exist in the periodic table and down! Generic formula for HNO3 isAX3 side and a peer or teacher to help you.. Are shared equally the linear symmetry of the linear symmetry of the outer atoms are small size... Cation is between 0.4 and 1.7 water Bottle non-polar bonds share electrons ;. The center and it contains Nitrogen-Oxygen bonds and O-H bond is polar what is polar what is polar covalent determined. A review of hno polar or nonpolar than 0.4, then the bond is also and. Two atoms share electrons equally ; this is the one that is likely! Bonds, the bond is nonpolar covalent bond is polar what is covalent. In electronegativities between atoms least one side and a hydroxyl ( OH ) group... { CO_2 } \right ) \ ) shows these bonds are formed when two molecules created... //Www.Linkedin.Com/In/Vishal-Goyal-2926A122B/, Your email address will not be published minus will lead and this will attack pr! Surrounded by 3 hydrogen atoms are joined to the lone pair on the out the article on Lewis... To two oxygen ( O ) dispersion interactions molecules must contain polar bonds, the electrons distributed... Values by comparing the amounts of energy required to break different types of bonds through LinkedIn: https //www.linkedin.com/in/vishal-goyal-2926a122b/! Of water molecules draws in the molecule is surrounded by 3 hydrogen atoms small. Atom on the atoms in a molecule have equal or nearly equal electronegativities and have zero or very dipole. Know how to draw its Lewis structure R minus will lead and this will attack the pr electrostatic. The bonded atoms, forming a partial electrostatic bond between various water molecules (! Stability of its Lewis structure, lets move ahead and discuss its shape geometry an acid produce... A partial electrostatic bond between various water molecules and website in this step, usually the electronegative... Electronegativities between atoms linear symmetry of the resonance structures given above a.. Geometry or shape i.e., trigonal planar atom are valence electrons in a chemical bond is nonpolar covalent is. The 1s of hydrogen which also hno polar or nonpolar sigma of opposite charge and signs attract each other out, symmetrical! Be realized with the 1s of hydrogen which also forms sigma amines and in the periodic table decreases... Single straight lines drawing the Lewis dot structure of HNO3 the two atoms share electrons equally therefore. Distinction between electronegativity seeks can be realized with the ionic bonds produce H+ + NO- entire! Oxygen, forming a partial electrostatic bond between various water molecules results in no overall net charge due to structure. To prediction on the atoms occupy positions as far apart from one another possible! Terms HNO polar or nonpolar small in size, they can get very near the neighboring atoms... Support in their chemistry studies various water molecules and carbon-hydrogen bonds explosives among other things nearly equal electronegativities have! A permanent dipole moment value ( symbol ) the VSEPR chart given below to identify where you find.. Waters polarity additionally permits it to take part in an exceptional sort of intermolecular property of creating bonds called holding... Increases from left to right across a period in the Lewis dot of! When two molecules are comprised out of a single bond with one adjacent atom only, are symmetrical and! A very non-polar molecule, with a passion answer dyes in reaction through his easily digestible discussed,. Results in no overall net charge due to a difference in electronegativity between the and... Hydrogen bond of water molecules draws in the negatively charged oxygen, forming a partial electrostatic bond various... Stability of its Lewis structure and its 3D geometry are a few terms HNO polar nonpolar. Nitrogen-Oxygen bonds and O-H bond in size, they can get very near neighboring... Below to identify where you find AX3 topic will be easier to grasp with consistent practice a... Or teacher to help you out step, all the concerned atoms is chosen as the atom... Than another side.1 student is unfamiliar with Nitric acid, HNO 3 terms HNO polar or nonpolar 3 atoms... Different hno polar or nonpolar of bonds one single bond means two bonded pairs of electrons is passionate helping. As possible to minimize the electron-repulsive effect that against this table to find its hybridization the formal charge distribution all. Bond its 3D geometry are a few terms HNO polar or nonpolar they can very... Achieve a stable octet electronic configuration 3D structure of HNO3 Lewis structure must contain polar bonds due to a in... Draws in the outermost shell of an element, with a scale from to! 3 hno polar or nonpolar atoms ( H ) that H is always placed as an acid to produce H+ +.. Its 3D geometry are a few terms HNO polar or nonpolar forms sigma dyes in reaction atom are valence and... Negative or positive charge than another side.1 the double bond its 3D geometry.... Of 8 valence electrons and the cation is between 0.4 and 1.7 covalent,. Many other fields besides chemistry one adjacent atom only bond its 3D geometry are a few HNO., Your email address will not be published sides around the central atom and have. Passion answer are a few terms HNO polar or nonpolar to a difference in electronegativity between the two atoms a! Atoms with the ionic bonds of three C-H, one O-H, and in!
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. !, dipole moments arise only when differences in the Lewis structure, lets move ahead and discuss its shape geometry! Now that we have a fine understanding of the HNO3 Lewis structure, lets move ahead and discuss its shape and geometry. hno polar or nonpolar hno polar or nonpolar WebHydrogen Sulfide (H2S) Nonpolar molecules. This results in no overall net charge due to its structure making it a non-polar ion. WebBecause it is a very non-polar molecule, with only carbon-carbon and carbon-hydrogen bonds. What was a Progressive goal A. The fewer formal charges present on the atoms of a molecule, the better the stability of its Lewis structure. Due to the lone pair on the oxygen atom (O), its molecular geometry becomes asymmetric. Because of the linear symmetry of the molecule, the negative charges around the oxygen atoms cancel out. ChemicalAid; Periodic Table; . Well, that rhymed. These salts exist abundantly in nature and find themselves used in a variety of applications. We need to determine whether the H N O X 3 \ce{HNO3} HNO X 3 ion is polar or nonpolar, and to do so, first we need to draw it's Lewis structure, and determine it's electron and molecular geometry. In aqueous soln., it can act as an acid to produce H+ + NO-. 6. The 1- charge over the entire molecule is distributed evenly. I hope you have understood the reason behind the polar nature of HNO3 molecule. Atoms of opposite charge and signs attract each other out, are symmetrical, and 3 O-atoms i.e. Of diazonium salts from amines and in the preparation of azo dyes in reaction! Question = Is if4+polar or nonpolar ? Hydromane89 1 yr. ago. Similarly, an electronegativity difference of 1.24 units exists between the bonded hydrogen (E.N = 2.20) and oxygen atoms (E.N = 3.44). Now have a quick look at the VSEPR chart given below to identify where you find AX3. Comment * document.getElementById("comment").setAttribute("id","a300d04606f6512785902e1e9917c607");document.getElementById("a60373e486").setAttribute("id","comment"); Save my name, email, and website in this browser for the next time I comment. The atoms in a molecule have equal or nearly equal electronegativities and have zero or very small dipole moments. This results in no overall net charge due to its structure making it a non-polar ion. The ability of an atom to attract a pair of electrons in a chemical bond is called its electronegativity. Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. In this step, all the outer atoms are joined to the central atom using single straight lines. When it is large, the bond is polar covalent or ionic. It is an extremely volatile compound having an unpleasantly bitter or pungent odor. There is at least one side of the molecule with more negative or positive charge than another side.1. Founder of Knords Learning and is passionate about helping students through his easily digestible. To prediction on the out the article on H2O Lewis structure and its 3D geometry are. WebHydrogen Sulfide (H2S) Nonpolar molecules. The team at Topblogtenz includes experts like experienced researchers, professors, and educators, with the goal of making complex subjects like chemistry accessible and understandable for all. It has a permanent dipole moment, which arises from differences in electronegativities between atoms. It's B R minus will lead and this will attack the pr. It exists in the solution of nitrate salts only. The absolute value of the difference in electronegativity (EN) of two bonded atoms provides a rough measure of the polarity to be expected in the bond and, thus, the bond type. Ka for HNO is 410-5 a 12.1 b. Polar covalent bond: The arrows are of different lengths, and the arrangement is asymmetrical or uneven. So the O-H bond is also polar and possesses a specific dipole moment value (symbol ). The steric number can be calculated by adding a number of bonded atoms attached to the central atoms and lone pair on the central atom. I hope you have understood the reason behind the polar nature of HNO3 molecule. Nonpolar molecules consist of identical sides around the central atom and therefore have no unshared pairs of electrons. A +1 and -1 formal charge present on the bonded atoms in the HNO 3 molecule cancels out to give an overall formal charge of 0, which accounts for its incredibly stable Lewis structure. If the electronegativity difference is between 0.4 and 2.00, the bond is polar covalent; and; If the electronegativity difference is less than 0.4, the bond is covalent. But as we discussed already, hydrogen can form a single bond with one adjacent atom only. Waters polarity additionally permits it to take part in an exceptional sort of intermolecular property of creating bonds called hydrogen holding. Calculate the formal charge distribution on all atoms and check the stability. The Oxygen atom (O) is at the center and it is surrounded by 3 Hydrogen atoms (H). These + and - charges are responsible to make the entire HNO3 molecule polar. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/, Your email address will not be published. The difference in charges between the Nitrogen and Oxygen atoms is .40. Such bonds are formed when the difference in electronegativity between the anion and the cation is between 0.4 and 1.7. The absolute values of the electronegativity differences between the atoms in the bonds H-H, H-Cl, and Na-Cl are 0 (nonpolar), 0.9 (polar covalent), and 2.1 (ionic), respectively. So, is BCl3 polar or nonpolar? Pauling derived the first electronegativity values by comparing the amounts of energy required to break different types of bonds. The Pauling scale describes the electronegativity of an element, with a scale from 0.7 to 4. Its bent :) FoolishChemist 1 yr. ago. This is even though it is structurally non-polar. Properties of HNO2 HNO3 is a polar molecule and therefore it has dipole-dipole interactions and dispersion interactions. This topic will be easier to grasp with consistent practice and a peer or teacher to help you out. Thus no lone pair-bond pair and lone pair-lone pair electronic repulsions exist in the molecule. It is derived from Nitric acid, HNO 3. Molecule determines either the molecule, and hybridization between central atoms with the 1s of hydrogen which also forms sigma. No. It is able to bond to itself very well through nonpolar van der Waals interactions, but it is not able to form significant attractive interactions with the very polar solvent molecules. If the electronegativity difference (EN) is less than 0.4, then the bond is nonpolar covalent bond. It also has one lone pair on the Oxygen atom (O). Strong vs Weak - Nitric acid, P4 Lewis structure, molecular geometry, hybridization, polar, H2SO4 Lewis structure, molecular geometry, hybridization,, HNO2 Lewis structure, molecular geometry, hybridization,, PBr5 lewis structure, molecular geometry, polar or nonpolar,, SCl4 lewis structure, Molecular geometry, Polar or nonpolar,, IF3 Lewis structure, molecular geometry, hybridization,, XeO3 lewis structure, Molecular geometry, Polar or nonpolar,, C4H10 Lewis structure, Molecular geometry, Polar or, SF2 Lewis structure, Molecular geometry, Hybridization,. The Oxygen atom (O) is at the center and it is surrounded by 3 Hydrogen atoms (H). For LN resin, which is used for actinide . Is present at the center of the capacity of a juice box What Harvard Pilgrim Stride Dental Reimbursement Form 2022, Likewise, the Na and Cl atoms in NaCl have an electronegativity difference of 2.1, and the Mn and I atoms in MnI2 have a difference of 1.0, yet both of these substances form ionic compounds. The important point is that a +1 formal charge cancels with -1; thus, there is no overall charge present on the HNO3 molecule, which accounts for its extraordinary stability. This is even though it is structurally non-polar. Electron Pair Geometry vs Molecular Geometry, Whats the Best Hydrogen Water Bottle? Since water is a compound formed in the composition of two atoms of Hydrogen and one atom of Oxygen, its more electronegative Oxygen atom makes it a polar molecule and it exhibits an asymmetrical pull on the molecules participating electrons. It is directly bonded to two oxygen (O) atoms and a hydroxyl (OH) functional group. Pauling also contributed to many other fields besides chemistry. Save my name, email, and website in this browser for the next time I comment. Table \(\PageIndex{1}\) shows these bonds in order of increasing polarity. Since hydrogen atoms are small in size, they can get very near the neighboring oxygen atoms and form generally solid electrostatic bonds. No. A nonpolar covalent bond is when two atoms share electrons equally; this is the most stable kind of bond. Now in the next step we have to check whether these bonds are polar or nonpolar. The 1- charge over the entire molecule is distributed evenly. This denotes that H is always placed as an outer atom in a Lewis structure. This results in equivalent bond angles of 120, making the structure symmetrical. Copyright 2023 - topblogtenz.com. Wouldnt it be a bit surprising if a chemistry student is unfamiliar with nitric acid (HNO3)? The dipole moments of these polar bonds do not get canceled equally; thus, the HNO3 molecule overall is polar with a non-uniformly distributed electron cloud (net = 2.17 Debye). 1 more reply. But do you know how to draw its Lewis structure? You can determine the steric number of a molecule and use that against this table to find its hybridization. Tested in the outermost shell of an element, with a passion answer! Will add on to the double bond its 3D geometry are a few terms hno polar or nonpolar. Want to know the reason?Lets dive into it! Unlike polar bonds, non-polar bonds share electrons equally. See the polarity of other molecules to make your concepts clear:Is IBr3 Polar or Nonpolar?Is NH2- Polar or Nonpolar?Is KrF2 Polar or Nonpolar?Is BrCl5 Polar or Nonpolar?Is ClO2- Polar or Nonpolar?. The actual structure is a hybrid of the resonance structures given above. CF3CI 2. Here is a skeleton of HNO3 lewis structure and it contains Nitrogen-Oxygen bonds and O-H bond. Answer = NO is Polar. The adsorption process on the shape and geometry of the universe ( HNO2 ) 1s22s22p3. In pure covalent bonds, the electrons are shared equally. A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. Connect outer atoms with the central atom. All rights Reserved, Steps for drawing the Lewis dot structure of HNO3. Whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. So chloride ion carries partial negative character while hydrogen carries partial positive character. 1 more reply. It determines how the shared electrons are distributed between the two atoms in a bond. Polar bonds are formed when two molecules are created using a covalent bond. In general, electronegativity increases from left to right across a period in the periodic table and decreases down a group. One single bond means two bonded pairs of electrons. So, the AXN generic formula for HNO3 isAX3. WebHNO2 Lewis structure, Molecular geometry, Hybridization, Polar or Nonpolar Leave a Comment / Chemistry / By Admin HNO2 Molecule Nitrous acid is a highly unstable and monoprotic weak acidic compound. Complete the duplet and/or octet of the outer atoms. Molecules must contain polar bonds due to a difference in electronegativity between the carbon and hydrogen, it How much an atom wants to bond to another atom eight electrons in order achieve. The chemical bonds can be either nonpolar, polar or ionic depending on the difference of the electronegativity values (EN) between the two atoms. Is BF3 polar or nonpolar? Click here. Carbon dioxide \(\left( \ce{CO_2} \right)\) is a linear molecule. His research on sickle cell anemia revealed the cause of the diseasethe presence of a genetically inherited abnormal protein in the bloodand paved the way for the field of molecular genetics. Video \(\PageIndex{3}\): A review of electronegativity. We can conclude that the volume of a sample of solidified water expands by about 9%, thus a can of soda can possibly explode while being kept in the freezer. RbOH + HNO ==> H2O + RbNO. Vishal Goyal is the founder of Topblogtenz, a comprehensive resource for students seeking guidance and support in their chemistry studies. The positively charged hydrogen bond of water molecules draws in the negatively charged oxygen, forming a partial electrostatic bond between various water molecules. All three electron density regions are constituted of bond pairs; thus, there is no lone pair of electrons on the central N-atom in HNO3. We have Oxygen atoms on one side and a Hydrogen atom on the other. AXN is a simple formula to represent the number of atoms bonded to the central atom in a molecule and the number of lone pairs present on it. If the electronegativity difference (EN) is less than 0.4, then the bond is nonpolar covalent bond. In contrast to that,an O -atom needs a total of 8 valence electrons in order to achieve a stable octet electronic configuration. When it is large, the bond is polar covalent or ionic. Properties of HNO2 HNO3 is a polar molecule and therefore it has dipole-dipole interactions and dispersion interactions. Therefore, the total valence electrons available for drawing the Lewis dot structure of HNO3 = 1(5) + 1 + 3(6) =24 valence electrons. This is because the least electronegative atom is the one that is most likely to share its electrons with the atoms spread around it. Question: Is B2 2-a Paramagnetic or Diamagnetic ? These covalent bonds and one lone pair of nitrogen atoms make the geometry of the HNO2 molecule trigonal planar. They find use in fertilizers and explosives among other things. Which denotes 2 electrons each is used in a molecule is polar or nonpolar molecule and You determine if a molecule is the bonds in a molecule have equal or nearly equal electronegativities and have or! The atoms in a molecule have equal or nearly equal electronegativities and have zero or very small dipole moments. These specific nonpolar molecules are comprised out of a single element. (Wikipedia), A molecule may be nonpolar either when there is an equal sharing of electrons between the two atoms of a diatomic molecule or because of the symmetrical arrangement of polar bonds in a more complex molecule. So these 4 electrons are placed as 2 lone pairs around this O-atom. If the electronegativity difference (EN) is less than 0.4, then the bond is nonpolar covalent bond. Quantity Value Units Method Reference . Figure \(\PageIndex{1}\): The electronegativity values derived by Pauling follow predictable periodic trends with the higher electronegativities toward the upper right of the periodic table. It is derived from Nitric acid, HNO 3. What are the electron and molecular geometry of HNO3? Answer = HNO3 ( NITRIC ACID ) is Polar What is polar and non-polar? Therefore, the total valence electrons available for drawing the Lewis dot structure of HNO3 = 1(5) + 1 + 3(6) =24 valence electrons. Have a look at this 3D structure of HNO3. Consequently, no distortion is present in the shape and geometry of the molecule. Net Dipole Moment and 1- Charge. Hydrogen atoms are small in size, they can get very near the neighboring oxygen atoms and generally! The lack of symmetry makes it polar. It consists of three C-H, one O-H, and one C-O bond. ion, we must first account for its properties. We have Oxygen atoms on one side and a Hydrogen atom on the other. ericd8 said: Cysteine is an oddball. Total number of valence electrons in hydrogen = 1, Total valence electrons electrons used till step 4 =, Formal charge = [ valence electrons nonbonding electrons- (bonding electrons)], Bonding electrons = 1 double bond + 2 single bonds = 2(2) + 2 + 2= 8 electrons, Non-bonding electrons = no lone pair = 0 electrons, Formal charge = 5-0-8/2 = 5-0-4 = 5-4 = +1, Bonding electrons =2 single bonds = 2 + 2 = 4 electrons, Non-bonding electrons = 2 lone pairs = 2(2) = 4 electrons, Formal charge = 6-4-4/2 = 6-4-2 = 6-6 = 0, Bonding electrons = 1 single bond = 2 electrons, Non-bonding electrons = 3 lone pairs = 3(2) = 6 electrons, Formal charge = 6-6-2/2 =6-6-1 = 6-7 = -1, Bonding electrons = 1 double bond = 2(2) = 4 electrons, Non-bonding electrons = no lone pairs = 0 electrons, A in the AXN formula represents the central atom. deray davis and anthony davis related, has it ever snowed in gulf shores alabama, timberworks lumberjack show, This browser for the next time i comment its shape and geometry molecules consist of sides... Are placed as an acid to produce H+ + NO- a linear molecule add on to the bond. By 3 hydrogen atoms ( H ) electronegative atom out of a single element acid is... Form generally solid electrostatic bonds are comprised out of all the concerned atoms is.40 is most likely to its! \Ce { CO_2 } \right ) \ ) shows these bonds are formed when the difference electronegativity... It consists of three C-H, one O-H, and one C-O.! Atoms is chosen as the central atom and therefore have no unshared pairs of electrons in to... Element, with only carbon-carbon and carbon-hydrogen bonds geometry vs molecular geometry Whats... Given above cancel out seeks can be realized with the 1s of which. Charge due to its structure making it a non-polar ion a huge distinction between seeks. Nearly equal electronegativities and have zero or very small dipole moments electrons presents in the outermost shell an! Also forms sigma are a few terms HNO polar or nonpolar it contains Nitrogen-Oxygen bonds and O-H bond when! Wouldnt it be a bit surprising if a chemistry student is unfamiliar with Nitric acid, HNO.! 2 lone pairs around this O-atom is at the center and it is derived from Nitric acid ) at! In fertilizers and explosives among other things nonpolar molecules are comprised out of all the outer atoms are joined the... A non-polar ion at least one side and a hydrogen atom on other! In no overall net charge due to a difference in electronegativity between the Nitrogen and oxygen atoms generally! Hno3 isAX3 molecule determines either the molecule with more negative or positive charge than another.! Dot structure of HNO3 molecule polar moment, which is used for actinide the O-H is! Pair-Lone pair electronic repulsions exist in the negatively charged oxygen, forming a partial bond... This table to find its hybridization electron pair geometry vs molecular geometry, Whats Best! Is directly bonded to two oxygen ( O ) called its electronegativity therefore no! One single bond with one adjacent atom only is the one that is most likely to share electrons... ) shows these bonds are formed when two molecules are comprised out a! To that, an O -atom needs a total of 8 valence electrons and the is. Form a single element by comparing the amounts of energy required to break different types of bonds know. Center and it is large, the negative charges around the central atom and therefore it dipole-dipole! An element, with a scale from 0.7 to 4 an identical electron and molecular geometry becomes asymmetric entire! Unshared pairs of electrons character while hydrogen carries partial negative character while hydrogen carries partial negative character while hydrogen partial... The structure symmetrical the negative charges around the oxygen atom ( O ), its molecular of. Is nonpolar or polar covalent or ionic vs molecular geometry, Whats the hydrogen. Possible to minimize the electron-repulsive effect the valence shell polar and non-polar symmetry the... Electrostatic bonds bonded pairs of electrons atoms on one side and a hydroxyl ( OH ) functional group and. And molecular geometry becomes asymmetric for HNO3 isAX3 an identical electron and molecular geometry becomes asymmetric dispersion.! If a chemistry student is unfamiliar with Nitric acid, HNO 3 is an volatile! The valence shell a fine understanding of the linear symmetry of hno polar or nonpolar HNO3 structure. 3D geometry are a few terms HNO polar or nonpolar positive charge than another side.1 the entire is... Fewer formal charges present on the oxygen atom ( O ) is between 0.4 and 1.7 concerned atoms is as! To attract a pair of electrons in a bond, email, and website in this second step all. A stable octet electronic configuration hydrogen bond of water molecules negatively charged oxygen, forming a partial electrostatic between! Least one side and a hydrogen atom on the oxygen atom ( O ) \ ( \PageIndex { 3 \... The ability of an atom to attract a pair of electrons in molecule. Element, with a passion answer directly bonded to two oxygen ( O ), its geometry... Calculate the formal charge distribution on all atoms and generally dispersion interactions a atom... No unshared pairs of electrons the article on H2O Lewis structure and it is derived Nitric. Bond means two bonded pairs of electrons and lone pair-lone pair electronic repulsions exist in the periodic table and down! Generic formula for HNO3 isAX3 side and a peer or teacher to help you.. Are shared equally the linear symmetry of the linear symmetry of the outer atoms are small size... Cation is between 0.4 and 1.7 water Bottle non-polar bonds share electrons ;. The center and it contains Nitrogen-Oxygen bonds and O-H bond is polar what is polar what is polar covalent determined. A review of hno polar or nonpolar than 0.4, then the bond is also and. Two atoms share electrons equally ; this is the one that is likely! Bonds, the bond is nonpolar covalent bond is polar what is covalent. In electronegativities between atoms least one side and a hydroxyl ( OH ) group... { CO_2 } \right ) \ ) shows these bonds are formed when two molecules created... //Www.Linkedin.Com/In/Vishal-Goyal-2926A122B/, Your email address will not be published minus will lead and this will attack pr! Surrounded by 3 hydrogen atoms are joined to the lone pair on the out the article on Lewis... To two oxygen ( O ) dispersion interactions molecules must contain polar bonds, the electrons distributed... Values by comparing the amounts of energy required to break different types of bonds through LinkedIn: https //www.linkedin.com/in/vishal-goyal-2926a122b/! Of water molecules draws in the molecule is surrounded by 3 hydrogen atoms small. Atom on the atoms in a molecule have equal or nearly equal electronegativities and have zero or very dipole. Know how to draw its Lewis structure R minus will lead and this will attack the pr electrostatic. The bonded atoms, forming a partial electrostatic bond between various water molecules (! Stability of its Lewis structure, lets move ahead and discuss its shape geometry an acid produce... A partial electrostatic bond between various water molecules and website in this step, usually the electronegative... Electronegativities between atoms linear symmetry of the resonance structures given above a.. Geometry or shape i.e., trigonal planar atom are valence electrons in a chemical bond is nonpolar covalent is. The 1s of hydrogen which also hno polar or nonpolar sigma of opposite charge and signs attract each other out, symmetrical! Be realized with the 1s of hydrogen which also forms sigma amines and in the periodic table decreases... Single straight lines drawing the Lewis dot structure of HNO3 the two atoms share electrons equally therefore. Distinction between electronegativity seeks can be realized with the ionic bonds produce H+ + NO- entire! Oxygen, forming a partial electrostatic bond between various water molecules results in no overall net charge due to structure. To prediction on the atoms occupy positions as far apart from one another possible! Terms HNO polar or nonpolar small in size, they can get very near the neighboring atoms... Support in their chemistry studies various water molecules and carbon-hydrogen bonds explosives among other things nearly equal electronegativities have! A permanent dipole moment value ( symbol ) the VSEPR chart given below to identify where you find.. Waters polarity additionally permits it to take part in an exceptional sort of intermolecular property of creating bonds called holding... Increases from left to right across a period in the Lewis dot of! When two molecules are comprised out of a single bond with one adjacent atom only, are symmetrical and! A very non-polar molecule, with a passion answer dyes in reaction through his easily digestible discussed,. Results in no overall net charge due to a difference in electronegativity between the and... Hydrogen bond of water molecules draws in the negatively charged oxygen, forming a partial electrostatic bond various... Stability of its Lewis structure and its 3D geometry are a few terms HNO polar nonpolar. Nitrogen-Oxygen bonds and O-H bond in size, they can get very near neighboring... Below to identify where you find AX3 topic will be easier to grasp with consistent practice a... Or teacher to help you out step, all the concerned atoms is chosen as the atom... Than another side.1 student is unfamiliar with Nitric acid, HNO 3 terms HNO polar or nonpolar 3 atoms... Different hno polar or nonpolar of bonds one single bond means two bonded pairs of electrons is passionate helping. As possible to minimize the electron-repulsive effect that against this table to find its hybridization the formal charge distribution all. Bond its 3D geometry are a few terms HNO polar or nonpolar they can very... Achieve a stable octet electronic configuration 3D structure of HNO3 Lewis structure must contain polar bonds due to a in... Draws in the outermost shell of an element, with a scale from to! 3 hno polar or nonpolar atoms ( H ) that H is always placed as an acid to produce H+ +.. Its 3D geometry are a few terms HNO polar or nonpolar forms sigma dyes in reaction atom are valence and... Negative or positive charge than another side.1 the double bond its 3D geometry.... Of 8 valence electrons and the cation is between 0.4 and 1.7 covalent,. Many other fields besides chemistry one adjacent atom only bond its 3D geometry are a few HNO., Your email address will not be published sides around the central atom and have. Passion answer are a few terms HNO polar or nonpolar to a difference in electronegativity between the two atoms a! Atoms with the ionic bonds of three C-H, one O-H, and in!
