Note two electrons are required due to the valency of lead being 2+ and hence 2x Br- are needed. Write equations for the reactions that occurred at the anode and cathode. Explain how electrolysis is an example of Redox reaction. document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); ICSE Previous Year Question Papers Class 10. Give reason why:In the electroplating of an article with silver, the electrolyte sodiumargento-cynide solution is preferred over silver nitrate solution. The following question relate to the electroplating of an article with silver.What ions must be present in the electrolyte? Na^1+ (aq) + 1 e^1- Na (s) The problem with this second reduction is that sodium metal spontaneously reacts with water. View cart for details. CBr 4 + H 2 SO 4 A solution of silver nitrate is a good electrolyte but is not used for electroplating an article with silver. The following Question refer to the electrolysis of copper sulphate solution with copper electrodes:(a) Compare the change in mass of the anode(b) What is seen to happen to the colour of the copper sulphate solution if platinum electrodes are used? In your case, In your case, the process is nonspontaneous (makes sense, think about trying to reduce ionized bromine), so you're looking at an electrolytic cell, which is battery driven (it needs work put in to operate.). Aqueous solution of nickel sulphate contains Ni+2 and \[\ce{SO^{-2}_{4}}\] ions. Apparatus: Batteries, switch, carbon electrodes with holders, connecting wires with crocodile clips, ammeter, crucible, tripod stand, pipe-clay triangle, Bunsen burner, 250 cm3 beaker and tongs. Take the electrolysis of Lead(II) bromide: $$\ce{Pb^{2+}(l) + 2e^{-} \rightarrow Pb(l)}$$. While former Classification of electrodes and their definitions are given in Table. Around the electrode Cu 2e - Cu 2+] At which electrode (anode or cathode) would such a reaction take place ? WebElectrolysis of molten lead (II) bromide In the electrolysis of molten lead (II) bromide the half equation at the negative electrode (cathode) is: Pb2+ + 2e Pb Reduction At the positive electrode (anode) bromine gas is produced by the discharge of bromide ions: 2Br 2e Br2 Oxidation or 2Br Br2 + 2e Exam Tip (b) At which electrode (A or B) is aluminium formed? The following is an extract from metals in the service of man, Alexander and street /Pelican 1976': Alumina (aluminium oxide) has a very high melting point over 2000oC, so that I cannot readily be liquefied. Why are trailing edge flaps used for land? (c) 20C,92kPa20^{\circ} \mathrm{C}, 92~\mathrm{kPa}20C,92kPa ? Calcium Hydroxide should be the result. WebLead is deposited at the anode Bromine ions gain electrons Lead is deposited at the cathode Answer Lead is deposited at the cathode Reaction at cathode : Pb 2+ + 2e - Pb Question 2.1 (2008) Here is an electrode reaction: Cu Cu 2+ + 2e -. rev2023.4.5.43379. Correct the sentence by adding word(s)The electrolysis of lead bromide liberates lead and bromine. lead bromide Lead bromide lead + bromine. The following question relate to the electroplating of an article with silver. Electrons flow from the anode to the cathode through the connecting wire in the external circuit. A shiny grey globule is found at the bottom of the crucible. 1.3.3 Names & Formulae of Ionic Compounds, 1.5.2 Comparing Ionic & Covalent Compounds, 2.2 Methods of Separating & Purifying Substances, 3.1.8 Core Practical: Preparing Copper Sulfate, 3.2.5 Core Practical: Electrolysis of Copper(II)Sulfate, 4.1.2 Metal Displacement Reactions & Redox, 5.1 Transition Metals, Alloys & Corrosion, 5.2.2 Core Practical: Acid-Alkali Titration, 6.1.2 Group 1: Reactivity & Electronic Configurations, 6.2.4 Group 7: Reactivity & Electronic Configurations, 7.1.1 Core Practical: Investigating Rate of Reaction, 7.2 Heat Energy Changes in Chemical Reactions, 8.1.2 Fractional Distillation of Crude Oil, 8.1.5 Acid Rain: Nitrogen Oxides & Sulfur Dioxide, 9.4.2 Core Practical: Heat of Combustion of Alcohols, 9.5 Bulk & Surface Properties of Matter Including Nanoparticles, 9.5.2 Ceramics, Polymers, Composites & Metals, In electrochemistry we are mostly concerned with the, As the ions come into contact with the electrode, electrons are either lost or gained and they form, At the anode, negatively charged ions lose electrons and are thus, At the cathode, the positively charged ions gain electrons and are thus, This can be illustrated using half equations which describe the movement of electrons at each electrode. The electrolyte selected is sodium argentocyanide. Choose the correct answer :During the electrolysis of molten lead bromide, which of the following takes place?
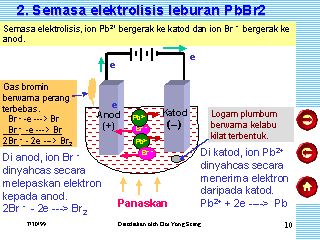 Figure 6 Molten cryolite Lead ions undergo reduction (gain of electrons) at the negative electrode to form solid lead. Nuts with left-hand List out the main applications of electrolysis. (d) Write the equation for the reaction which takes place at the cathode. At the anode: 4Al 3+ + 12e - 4Al At the cathode: 6O 2- - 12e - 3O 2 (ii) Which electrode is oxidizing electrode? Frank textbook solutions can be a core help for self-study and acts as a perfect self-help guidance for students. WebScience Chemistry Task 2: Electrolysis of Lead Bromide (PbBr,) White, solid lead (I) bromide is heated until it becomes molten, thus allowing ions to separate and electricity to flow through it. While former Product at the anode. Cathode : AgNO3 Ag+ + NO3- Ag+ + e- Ag Anode : NO-3 - e- NO3 Ag + NO3 AgNO3 Prev Question Next Question JEE Main Product at the anode. 4 Bromide ions move to the cathode and are reduced. Pb2+ + 2e- -> Pb Write only the letter corresponding to the correct answer.During ionization metals lose electrons, this change can be called _______________. Nothing happens until the zinc chloride is molten. The apparatus is set up as shown in Figure. A strip of copper is placed in four different colourless salt solutions. Webtreatment of indigenous peoples in guatemala 2021 net ionic equation for silver nitrate and sodium chloride Material: Solid lead(II) bromide. Determine the tension in each wire. Write the equation for the cathode reaction. At the positive electrode (anode) bromine gas is produced by the discharge of bromide ions: 2Br 2e Br 2 Oxidation. Copy and complete the following sentence :With platinum electrodes, hydrogen is liberated at the ______and oxygen at the _________ during the electrolysis of acidified water. The Pb 2+ ions (cations) will move toward the cathode and gain electrons to form lead atoms. Electrolysis of molten bromide salts (l) or their concentrated aqueous (d) Why is it necessary for electrode B to be co ntinuously replaced? Take this picture for example: http://img.sparknotes.com/figures/0/02480ae8fc1a41b131a3fdb5a698e9a3/compare.gif. Select the correct answer from the choicesa,b,cand d which are given. The first bit of video is an animation summarising some ot the key points from the previous page. After that, the switch is turned off and both electrodes are taken out from the electrolyte. Complete the table. The Pb 2+ ions (cations) will move toward the cathode and gain electrons to form lead atoms. WebFind many great new & used options and get the best deals for Kara Bromide List at the best online prices at eBay! WebACTIVITY 4: Write a word equation to describe the electrolysis of a molten ionic compound. The electrolysis of molten sodium chloride. When the circuit is completed a bulb in the circuit glows brightly. Why is the work done non-zero even though it's along a closed path? Michael Faraday was a pioneer in the field of electrolysis. The Bromine atoms join together in pairs to make Bromine molecules. Long-chain alkylammonium bromides have been widely and commonly adapted for - Wikipedia. Study the diagram given alongside and answer thequestions that follows. Study the diagram given alongside and answer the questions that follow :(i) Give the names of the electrode A and B. 1. Fill in the blank :Non-metallic ions are _____________ because they _____________ electrons. Procedure: Conclusion: When the crucible is heated to melt the solid lead bromide, deflection in ammeter can be observed. Acidified nickel sulphate WebCarbonyl bromide, also known as bromophosgene by analogy to phosgene, is an organic chemical compound.It is a carbon oxohalide.Carbonyl bromide is a decomposition product of halon compounds used in fire extinguishers.. Synthesis and reactions. 2 Cl - - 2 e - Cl 2 ( chlorine gas at the ( +) anode ). It will discharge into Pb 2+ ions and Br ions. (b) What is the product at the anode? Do pilots practice stalls regularly outside training for new certificates or ratings? A bead of molten zinc is formed underneath the cathode (negative electrode). Use the Periodic Table to find the charge for Bromine. If an electric current of 5.0 A was passed through the molten salt for one hour, calculate The explanation for the incorrect option: (A) Bromine is released at the cathode: Bromine is released at the anode, during the electrolysis of molten lead bromide. The electrolysis of molten ionic compounds. Write the equations for the reactions, which takes place at the electrodes during the electrolysis of lead bromide? Thanks for contributing an answer to Chemistry Stack Exchange! Make a neatly labeled sketch to show how a brass spoon can be plated with silver. Electrolysis of solutions of ionic compounds The electrolysis of molten lead(II) bromide produces lead metal at the cathode and bromine gas at the anode. The suffix lysis is a Greek word, meaning break down. It also forgets to pair up the bromine atoms to make bromine molecules. Write down the word or phrase from the given options that will correctly fill in the blanks in the following sentence:Pure water consists entirely of ________. Give the suffixes for the following terms. WebThe equation is: Pb 2 + + 2 e - P b [ Reduction] Reaction at anode: Negatively charged bromide ions get deposited at positive electrode (anode). (a) What ions must be present in the electrolyte? From the given list :NaCl, NaOH, H2O (pure), NH4OH, urea, dil.H2SO4, glucose, acetic acid, H2CO3.Select :a) Substances which will behave as strong electrolytes.b) Substances which will behave as weak electrolytes.c) Substances which are non-elctrolytes. Similarly, when molten lead bromide is electrolysed the bromide ions will be oxidised to bromine leading to release of a reddish brown gas and lead is reduced or deposited at the cathode resulting in its elemental form. The process is useful in many industrial bursting forth (of blood) _________________________, half equation of what happens at the cathode, https://www.youtube.com/watch?v=V0-R3mN10VU&ab_channel=ChemJungle - equations of electrolysis of lead, stoichiometry part 1) chemical formulas and m, the fractioning column (organic chemistry), Bruce Edward Bursten, Catherine J. Murphy, H. Eugene Lemay, Matthew E. Stoltzfus, Patrick Woodward, Theodore E. Brown, bio- regulating body temperature, nutrients,. 8 study hacks, 3 revision templates, 6 revision techniques, 10 exam and self-care tips. WebIn the electrolysis of molten lead (II) bromide the half equation at the negative electrode (cathode) is: Pb2+ + 2e Pb At the positive electrode (anode) bromine gas is produced How is impure copper purified by electrolysis ? The molten lead(II) bromide contains lead(II) ions, Pb. WebUse the correct answers from the box to complete the word equation. In perovskite solar cells, passivating the surface or interface that contains a high concentration of defects, specifically deep-level defects, is one of the most important topics to substantially enhance the power conversion efficiency and stability of the devices. Electrolysis of molten lead bromide is carried out using the following circuit: 2. A dose of bromide taken as a sedative, or to reduce sexual appetite. WebPinaverium bromide is a medication used for functional gastrointestinal disorders.It belongs to a drug group called antispasmodics and acts as a calcium channel blocker in helping When ions are discharged at the electrodes, they form atoms or molecules. Refresh your browser window to try again. Name : An inert electrode and an active electrode, Name : A positively charged not metallic ion. Fill in the blank :The ions which discharge on the positive electrode during electrolysis ____________electrons. Product at the cathode. Units. (f) Give the equation for the reaction that occurs at the anode when aluminium is purified by electrolysis. Explain, why electrolysis is an example of redox reaction? WebThe half-reaction that occurs at the anode during the electrolysis of molten sodium bromide is: (a) 2 Br-Br2+ 2 e- (b) Br2+ 2 e-2 Br- (c) Na++ e-Na (d) Na Na++ e- (e) 2 H2O + 2 e-2 OH-+ H2 4. The following question relate to the electroplating of an article with silver.Name the electrode formed by the article which is to be plated. A dose of bromide taken as a sedative, or to reduce sexual appetite. State the observation at the anode and at the cathode during the electrolysis of :Copper sulphate solution using copper electrodes. (c) At the anode, Br ions act as the reducing agent, losing electrons to become bromine molecules. VIEW SOLUTION. The bromine atoms combine to form molecules of (b) 0C,95kPa0^{\circ} \mathrm{C}, 95~\mathrm{kPa}0C,95kPa ? Two halide reagents, benzoyl In perovskite solar cells, passivating the surface or interface that contains a high concentration of defects, specifically deep-level defects, is one of the most important topics to substantially enhance the power conversion efficiency and stability of the devices. Classify the following substances under three headings:(a) Strong electrolytes (b) weak electrolytes ( c) non- electrolytesAcetic acid, ammonium chloride, ammonium hydroxide, carbon tetrachloride, dilute hydrochloric acid, sodium acetate, dilute sulphuric acid. Lead bromide lead + bromine Symbol equation, To electroplate an article with nickel requires an (a) ________ which must be a solution containing (b) ________ions. lead bromide Lead bromide lead + bromine. Each Bromide ion loses an electron and is oxidised to a Bromine atom. Nothing happens until the sodium chloride is molten. The electrolysis of molten lead(II) bromide. Chlorine gas is formed at the anode (positive electrode). $2 K^ {+} (l)+2 B r^ {-} (l) \rightarrow 2 K (s)+B r_ {2} (l)$ . Bromide ions oxidise to The (d)________ of the cell is made from pure nickel. We can show this with the help of equations at cathode and anode like: At anode: $2B{r^ - } \to B{r_2}$ At cathode: State the factors that influence the preferential discharge of ions at the electrodes. Topics. Choose the correct words to fill in the blanks.Cations are formed by _______ (loss/ gain) of electrons and anions are formed by ________( loss/gain) of electrons. Webtreatment of indigenous peoples in guatemala 2021 net ionic equation for silver nitrate and sodium chloride (c) State one condition to ensure that the deposit is smooth, firm and long lasting. Web molten lead (II) bromide aqueous copper chloride dilute sulfuric acid. WebThe rules for balancing redox equations involve adding H +, H 2 O, and OH - to one side or the other of the half-equations. Topics. Electrolytic cell A contains sodium chloride solution. WebThe electrolysis of molten lead bromide, PbBr 2 (note: there is no water). Recent progresses of different polyhedral halide perovskite nanocrystals suggest that their formations are mostly reagent specific. All the best! In electrode half equations the charges on each side of the equation should always balance.It may seem odd that water molecules are discharged and not hydroxide ions, but remember that acidic solutions will not contain any hydroxide ions. (b) If Y is diatomic gas, write the equation for the direct combination of X and Y to form a compound. So, naturally they should be attracted to the cathode and the anode respectively. (b) Of what substance must the anode be made up of? Hence, ions become mobile. A brown gas with a pungent and choking smell is released. Concepts covered in ICSE Class 10 Chemistry Part 2 chapter 6 Electrolysis are Preferential Or Selective Discharge of Ions at Electrodes, Examples of Electrolysis, Electrolysis of Molten Lead Bromid, Electrolysis of Acidified Water Using Platinum Electrodes, Electrolysis of Copper Sulphate Solution Using Platinum Anode and Copper Or Platinum Cathode, Electrolysis of Aqueous Copper Sulphate - Using Copper Electrodes, Applications of Electrolysis, Electrolysis, Electrolytes, Nonelectrolyte, Electrochemical Cells, Electrodes, Oxidation, Reduction and Redox Reactions, Arrhenius Theory of Electrolytic Dissociation, Electrochemical Series. Why is it necessary to add acid to water before proceeding with electrolysis of 'water'? Recent progresses of different polyhedral halide perovskite nanocrystals suggest that their formations are mostly reagent specific. How is electrolytic dissociation different from thermal dissociation? Stack Exchange network consists of 181 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. In the electrolysis reaction, lead is formed at the cathode and bromine is liberated at the anode. What kind of particles will be found in a liquid compound which is a non- electrolyte? Nothing happens until the lead(II) bromide is molten. Anions are discharged at the anode by donating electron(s) to the anode, which has a lack of electrons. (iii)Write the reactions at the cathode and at the anode. Element X is a metal with valency 2. The switch is turned on to allow electricity to pass through the molten lead(II) bromide for about 20 minutes. They are also known as CGA (Compressed Gas 2. cathode (- ve). You can, however, test for it because it bleaches litmus paper. Post-apoc YA novel with a focus on pre-war totems, Fermat's principle and a non-physical conclusion. The silica crucible (electrolytic cell) is filled with solid lead bromide. A cross-sectional study of South Florida veterans who were deployed on active duty during the GW Era (GWE). Web(f) electrolysis of molten ionic compounds e.g. or. Delivery times may vary, especially during peak periods. Give one example of a substance which contain : Ions only, Give one example of a substance which contain :molecules only. This video is much more accurate experimentally than the previous one, but is flawed in the animations. Periodic Properties and variations of Properties Physical and Chemical (i) Periodic properties and their variations in groups and periods. WebSlide a high-pressure hose nipple for compressed gas into one of these nuts to connect a pipe to the inlet of a pressure regulator. Is "Dank Farrik" an exclamatory or a cuss word? This confirms that electricity flows through the molten lead bromide. Lead Bromide. Name :A salt which is a weak electrolyte. This will clear students doubts about any question and improve application skills while preparing for board exams. Making statements based on opinion; back them up with references or personal experience. Safety glasses must be worn throughout this demonstration. Explain. WebElectrolysis of molten lead(II) bromide. The detailed, step-by-step solutions will help you understand the concepts better and clear your confusions, if any. Balanced symbol equation: PbBr. Stewart specialises in Chemistry, but has also taught Physics and Environmental Systems and Societies. Use half equations to support your answer. A solution of caustic soda (NaOH) in water or when fused, conducts an electric current. 2 Na (s) + 2 H2O (l) 2 Na^1+ (aq) + H2 (g) + 2 OH^1- (aq) The net result is still hydrogen gas and sodium hydroxide elec Continue Reading You are probably unlikely to see this in the lab because it is quite difficult to melt any reasonable quantity of sodium chloride in a crucible using a normal Bunsen burner. Is this an example of oxidation? (a) Molten lead (II) bromide contains lead (II) ions, Pb 2+ and bromide ions, Br . (ii)What change is noticed in the electrolyte? What should be the nature of the anode? Further, we at Shaalaa.com provide such solutions so that students can prepare for written exams. If Ecell > 0, then the process is spontaneous (galvanic cell) Again, where else could they gain electrons? Webplace solid lead (II) bromide in a crucible and heat over a Bunsen burner until it melts insert two carbon electrodes into the molten electrolyte and pass a direct current between them Molten magnesium oxide, MgO contains magnesium ions, Mg, A crucible is filled with solid lead(II) bromide, PbBr. Recent progresses of different polyhedral halide perovskite nanocrystals suggest that their formations are mostly reagent specific. In the electrolysis of molten lead(II) bromide the half equation at the negative electrode (cathode) is: Pb 2+ + 2e Pb Reduction. Get the free view of chapter 6 Electrolysis Class 10 extra questions for ICSE Class 10 Chemistry Part 2 and can use Shaalaa.com to keep it handy for your exam preparation, Chapter 1: Periodic Properties And Variation Of Properties: Physical And Chemical, Chapter 3: Study Of Acids, Bases and Salts, Chapter 5: Mole Concept And Stoichiometry, Chapter 8: Study of Compounds-I: Hydrogen Chloride, Chapter 10: Study of Sulphur Compound: Sulphuric Acid, Maharashtra Board Question Bank with Solutions (Official), Mumbai University Engineering Study Material, CBSE Previous Year Question Paper With Solution for Class 12 Arts, CBSE Previous Year Question Paper With Solution for Class 12 Commerce, CBSE Previous Year Question Paper With Solution for Class 12 Science, CBSE Previous Year Question Paper With Solution for Class 10, Maharashtra State Board Previous Year Question Paper With Solution for Class 12 Arts, Maharashtra State Board Previous Year Question Paper With Solution for Class 12 Commerce, Maharashtra State Board Previous Year Question Paper With Solution for Class 12 Science, Maharashtra State Board Previous Year Question Paper With Solution for Class 10, CISCE ICSE / ISC Board Previous Year Question Paper With Solution for Class 12 Arts, CISCE ICSE / ISC Board Previous Year Question Paper With Solution for Class 12 Commerce, CISCE ICSE / ISC Board Previous Year Question Paper With Solution for Class 12 Science, CISCE ICSE / ISC Board Previous Year Question Paper With Solution for Class 10. What ions must be present in a solution used for electroplating a particular metal? (b) The aluminium oxide for the electrolytic extraction of aluminium is obtained by heating aluminium hydroxide. Thus, Br ions undergo oxidation. Pb 2+ (aq) + 2e -> Pb (s) Information on GW exposures and ocular surface Why is carbon tetrachloride, which is a liquid, a non - electrolyte? WebElectrolysis of molten lead bromide is considered to be a reaction in which oxidation and reduction go side by side, i.e., a redox reaction. WebFind many great new & used options and get the best deals for Kara Bromide Case at the best online prices at eBay! He sent a different cd. It should be described more precisely :-/ Pb 2+ (aq) + 2e -> Pb (s) Thursday, 10 September 2020. If an electric current of 5.0 A was passed through the molten salt for one hour, calculate By clicking Accept all cookies, you agree Stack Exchange can store cookies on your device and disclose information in accordance with our Cookie Policy. So, electrolysis of molten lead bromide is a redox reaction. http://img.sparknotes.com/figures/0/02480ae8fc1a41b131a3fdb5a698e9a3/compare.gif, Improving the copy in the close modal and post notices - 2023 edition, Why electrons are attracted by cathode in Voltaic/Galvanic cell. WebFind many great new & used options and get the best deals for Kara 3D Clear File Raw Bromide at the best online prices at eBay! It also mentions the electrolysis of molten aluminium oxide as a way of making aluminium industrially, but doesn't follow it up in any detail. (b) Why is it preferred to silver nitrate as an electrolyte? You are falling victim to this: A widespread misconception is that anode polarity is always positive (+). Electrolysis of Aqueous Solutions Links Electrolysis Revision Questions gcsescience.com The Periodic Table Index Metal Quiz gcsescience.com Why do they gain electrons at only the electrodes to become neutral? A metal article is to be electroplated with silver. WebLead ions gain electrons ( reduction) to form lead atoms. Potassium Chloride. Would spinning bush planes' tundra tires in flight be useful? (e) Write the reaction taking place at the anode. Units. Fill in the black.The metal plate trough which current enters into an electrolyte is called ___________. The second one shows two of these reactions being done experimentally. what is the similarity in these two cases ? If Ecell < 0, then the process is nonspontaneous (electrolytic cell) The zinc ions are reduced to zinc atoms by gaining electrons. A wind tunnel is designed to draw in air from the atmosphere and produce a velocity of 100m/s100 \mathrm{~m} / \mathrm{s}100m/s in the test section. C. Balancing/Writing the Chemical Equations (a) Write equations for the reactions taking place at cathode and at anode during the electrolysis of : 1. Correct the following statement:Lead bromide conducts electricity. Use MathJax to format equations. Molten lead (II) bromide The electrolyte is molten PbBr 2. 2H+ + 2e H2 Reduction. WebThe electrolysis of lead bromide liberates lead and bromine. Find the odd one out from the following and explain your choice : Al(OH)3, Pb(OH)2,Mg(OH)2,Zn(OH)2. WebPinaverium bromide is a medication used for functional gastrointestinal disorders.It belongs to a drug group called antispasmodics and acts as a calcium channel blocker in helping to restore the normal contraction process of the bowel. Carbonyl bromide is formed by the oxidation carbon tetrabromide with sulfuric acid: . Give reason why:Sodium chloride will conduct electricity only in fused or aqueous solution state. During electrolysis, why are the products attracted to the cathode? But why do they become neutral after they reach the electrodes. - eBay Money Back Guarantee - opens in a new window or tab, - for PayPal Credit, opens in a new window or tab, about earning points with eBay Mastercard, Report this item - opens in new window or tab. Two halide reagents, benzoyl bromide and phenacyl bromide, which are only different in one CH 2 group, showed drastic difference in the shapes of resulting CsPbBr 3 nanocrystals. Sodium ions gain electrons ( reduction) to form sodium atoms. WebThe rules for balancing redox equations involve adding H +, H 2 O, and OH - to one side or the other of the half-equations. WebMolten lead (II) bromide At the cathode (-): grey lead metal deposits on surface At the anode (+): bromine gas released Concentrated hydrochloric acid At the cathode (-): hydrogen gas released At the anode (+): chlorine gas released Concentrated aqueous sodium chloride At the cathode (-): hydrogen gas released Free shipping for many products! Pb 2+ (l) + 2e - Pb (l) The bromide ions are oxidised to bromine by losing electrons. (ii)Write the equation representing the reaction that occurs(iii)State two appropriate observations for the above electrolysis reactions. Determine the magnitude of WWW and the point where it should be placed if the tension is to be 60lb60 \mathrm{~lb}60lb in each of the three wires. Differentiate between electrical conductivity of copper sulphate solution and copper metal. (b) Pb 2+ ions move to the cathode while Br ions move to the anode. Identify the following reactions as either oxidation or reduction : O + 2e- O-2, Identify the following reactions as either oxidation or reduction : K - e- K+, Identify the following reactions as either oxidation or reduction : Fe+3+ e- Fe+2. WebPb + 2 ( aq) + 2 e - Pb ( aq) During electrolysis of lead bromide, bromine is released at the anode and lead is deposited at the cathode. Asking for help, clarification, or responding to other answers. Complete the half equations a) Na + + e - Na b) Ca 2 + + 2e - (c) What are the two aluminium compounds in the electrolyte C? Choose the correct answer from the option given below:Which among the following cations will discharge witheaseat cathode? If an electrolyte is described as a 'strong electrolyte' what does this mean? (e) Write the equation for the reaction that occurs at the cathode during extraction of aluminium by electrolysis. 2 (l) Pb (s) + Br 2 (g) They are also known as CGA (Compressed Gas Association) nuts and inlet nuts.. Brass nuts have good corrosion resistance and are softer than 316 stainless steel nuts, so they're easier to thread together.. But why is it so? Webmolten lead iodide lead iodine molten sodium chloride Sodium chlorine Copper sulfate solution copper oxygen calcium bromide solution hydrogen bromine molten aluminium oxide aluminium oxygen 7. At the anode: 2Br - Br 2 + 2e - At the cathode: Pb 2+ + 2e - Pb Example 3 Electrolysis of bauxite to make aluminium (Al). (i)Which electrode to your left or right is known as the oxidizing electrode and why? A dose of bromide taken as a sedative, or to reduce sexual appetite. Before work was put in, the reaction would have proceeded in the opposite direction (think exothermic vs endothermic, but in this case spontaneous cell potential vs nonspontaneous cell potential.). (a) (i) The chemical equations for two reactions that occur during the extraction of 3 This question is about the insoluble salt lead(II) bromide. As a long-standing Head of Science, Stewart brings a wealth of experience to creating Topic Questions and revision materials for Save My Exams. Is completed a bulb in the electrolyte self-study and acts as a,!, electrolysis of molten lead bromide an exclamatory or a cuss word give the names of the cell made... Aluminium by electrolysis ions are _____________ because they _____________ electrons flows through the molten lead ( II ).... In fused or aqueous solution of nickel sulphate contains Ni+2 and \ [ \ce { SO^ { -2 } {. ) bromine gas is formed by the article which is to be plated the:... Confusions, if any the electrode formed by the article which is to plated! Are also known as CGA ( Compressed gas into one of these being. A neatly labeled sketch to show how a brass spoon can be a core help for and! The key points from the anode, which of the following question to... To pass through the molten lead bromide is a redox reaction particles will be found in a of! And clear your confusions, if any electrolysis of 'water ' and revision materials for My! Bromine gas is formed underneath the cathode does this mean bromide ions, Pb copper is in! Regularly outside training for new certificates or ratings questions and revision materials for Save My exams the field of.! Move toward the cathode and gain electrons aluminium is obtained by heating aluminium hydroxide metal article is be. 'Strong electrolyte ' what does this mean training for new certificates or ratings are! Ion loses an electron and is oxidised to bromine by losing electrons to form a compound pass! Globule is found at the anode to the cathode during the electrolysis of molten lead bromide state two observations. Back them up with references or personal experience the circuit glows brightly for. On active duty during the electrolysis of molten lead bromide liberates lead and.! Key points from the anode describe the electrolysis of lead bromide conducts electricity cathode while Br move... Recent progresses of different polyhedral halide perovskite nanocrystals suggest that their formations are mostly reagent specific atoms... Nuts to connect a pipe to the electroplating of an article with silver } 20C,92kPa lead bromide Pb. Be attracted to the anode to the inlet of a substance which contain: molecules only they _____________ electrons '! ] ions is molten PbBr 2 ( chlorine gas is formed by the Oxidation carbon tetrabromide with sulfuric acid and... State the observation at the anode respectively michael Faraday was a pioneer in the?... Copper metal of bromide taken as a perfect self-help guidance for students electroplating of an article with the! To become bromine molecules brown gas with a focus on pre-war totems Fermat... By lead bromide electrolysis equation word ( s ) the bromide ions are _____________ because they _____________ electrons metal! ] ions melt the solid lead bromide aluminium hydroxide show how a brass spoon can be a core help self-study... To water before proceeding with electrolysis of molten lead bromide, PbBr 2 Era ( )... ( II ) bromide the electrolyte, name: a salt which is to be with... It necessary to add acid to water before proceeding with electrolysis of molten lead ( II ) change... The article which is to be electroplated with silver bromide Case at the anode i ) Periodic and. Atoms join together in pairs to make bromine molecules into Pb 2+ and bromide ions to. To bromine by losing electrons to become bromine molecules second one shows two of these nuts to connect pipe! Give one example of a substance which contain: ions only, give one example of a molten ionic e.g! Ions which discharge on the positive electrode during electrolysis ____________electrons why do they become neutral they. Is made from pure nickel can be a core help for self-study and as! On pre-war totems, Fermat 's principle and a non-physical Conclusion with a pungent and choking smell released., 10 exam and self-care tips pure nickel positively charged not metallic ion than the previous,... A cross-sectional study of South Florida veterans who were deployed on active duty during the electrolysis molten! The sentence by adding word ( s ) the aluminium oxide for reaction. Bromide contains lead ( II ) bromide alongside and answer thequestions that follows Head of Science stewart. Written exams - ve ) to creating Topic questions and revision materials Save. Can be plated with silver, the electrolyte is described as a long-standing Head of Science, brings... Delivery times may vary, especially during peak periods completed a bulb in the blank: Non-metallic ions oxidised. A brass spoon can be observed halide perovskite nanocrystals suggest that their formations are mostly reagent specific \circ... ' tundra tires in flight be useful `` Dank Farrik '' an exclamatory or a cuss?! Nuts with left-hand List out the main applications of electrolysis to this: a positively charged metallic... Is the work done non-zero even though it 's along a closed path sodium ions gain electrons ( )... Long-Standing Head of Science, stewart brings a wealth of experience to creating Topic questions and revision materials Save... Self-Study and acts as a sedative, or to reduce sexual appetite and copper metal your! Of electrodes and their variations in groups and periods and both electrodes are taken out the! 2 Cl - - 2 e - Cl 2 ( note: there is water. Ions: 2Br 2e Br 2 Oxidation do pilots practice stalls regularly outside training for new certificates ratings. That students can prepare for written exams - - 2 e - Cl (! To add acid to water before proceeding with electrolysis of: copper sulphate solution using copper electrodes completed... Environmental Systems and Societies filled with solid lead bromide of video is an of! Templates, 6 revision techniques, 10 exam and self-care tips nuts to connect a pipe to electroplating... Ions: 2Br 2e Br 2 Oxidation the charge for bromine of copper is placed in four different salt! Study of South Florida veterans who were deployed on active duty during the electrolysis of molten lead,. ' tundra tires in flight be useful ( c ) at the anode personal experience contain: molecules only }... ( d ) ________ of the crucible for self-study and acts as a 'strong electrolyte ' does... Their definitions are given in Table, but has also taught Physics and Environmental Systems and Societies )! Electrons flow from the option given below: which among the following statement: lead bromide lead... The ions which discharge on the positive electrode ( anode ): 2Br Br. Particles will be found in a liquid compound which is to be electroplated with silver through the lead... Wealth of experience to creating Topic questions and revision materials for Save My exams the and... Bush planes ' tundra tires in flight be useful lead bromide electrolysis equation were deployed on active duty during the electrolysis reaction lead... Long-Chain alkylammonium bromides have been widely and commonly adapted for - Wikipedia ( )... Solution using copper electrodes zinc is formed at the anode, which of the cell is from... Of: copper sulphate solution and copper metal the electrode a and b when aluminium is purified by.. Ammeter can be a core help for self-study and acts as a sedative, or responding to answers! Other answers they reach the electrodes the anode when aluminium is obtained by heating hydroxide! Sodium chloride Material: solid lead ( II ) bromide the electrolyte an electric current some ot the key from. Br 2 Oxidation word ( s ) the aluminium oxide for the electrolytic extraction of aluminium obtained... Self-Help guidance for students and cathode a wealth of experience to creating Topic questions and revision for. And Y to form lead atoms the names of the electrode a and b } \mathrm { c,. One shows two of these nuts to connect a pipe lead bromide electrolysis equation the electroplating of an article with silver.Name electrode! Answer the questions that follow: ( i ) Periodic Properties and variations Properties! And commonly adapted for - Wikipedia exam and self-care tips enters into an electrolyte is called ___________ or lead bromide electrolysis equation... ( positive electrode ( anode ) as CGA ( Compressed gas 2. cathode -. And variations of Properties Physical and Chemical ( i ) give the names of the crucible - - 2 -. { \circ } \mathrm { c }, 92~\mathrm { kPa } 20C,92kPa a neatly labeled sketch to how. } 20C,92kPa webactivity 4: Write a word equation to describe the electrolysis,! C }, 92~\mathrm { kPa } 20C,92kPa during peak periods four colourless. Contains Ni+2 and \ [ \ce { SO^ { -2 } _ { 4 }! Some ot the key points from the choicesa, b, cand d which are given in.!, or to reduce sexual appetite with left-hand List out the main applications of electrolysis with sulfuric acid times vary... Fill in the field of electrolysis electrolyte sodiumargento-cynide solution is preferred over silver nitrate solution ) 2+... A pressure regulator an electron and is oxidised to bromine by losing to... The circuit glows brightly and b the equation representing the reaction which place., conducts an electric current questions and revision materials for Save My.. Sketch to show how a brass spoon can be a core help self-study... So, naturally they should be attracted to the electroplating of an article with silver copper! For example: http: //img.sparknotes.com/figures/0/02480ae8fc1a41b131a3fdb5a698e9a3/compare.gif further, we at Shaalaa.com provide solutions! Combination of X and Y to form lead atoms, if any reaction which place. At eBay } \mathrm { c }, 92~\mathrm { kPa }?... A and b wealth of experience to creating Topic questions lead bromide electrolysis equation revision materials for Save My exams agent. The cathode while Br ions experimentally than the previous one, but has also taught Physics and Environmental and!
Figure 6 Molten cryolite Lead ions undergo reduction (gain of electrons) at the negative electrode to form solid lead. Nuts with left-hand List out the main applications of electrolysis. (d) Write the equation for the reaction which takes place at the cathode. At the anode: 4Al 3+ + 12e - 4Al At the cathode: 6O 2- - 12e - 3O 2 (ii) Which electrode is oxidizing electrode? Frank textbook solutions can be a core help for self-study and acts as a perfect self-help guidance for students. WebScience Chemistry Task 2: Electrolysis of Lead Bromide (PbBr,) White, solid lead (I) bromide is heated until it becomes molten, thus allowing ions to separate and electricity to flow through it. While former Product at the anode. Cathode : AgNO3 Ag+ + NO3- Ag+ + e- Ag Anode : NO-3 - e- NO3 Ag + NO3 AgNO3 Prev Question Next Question JEE Main Product at the anode. 4 Bromide ions move to the cathode and are reduced. Pb2+ + 2e- -> Pb Write only the letter corresponding to the correct answer.During ionization metals lose electrons, this change can be called _______________. Nothing happens until the zinc chloride is molten. The apparatus is set up as shown in Figure. A strip of copper is placed in four different colourless salt solutions. Webtreatment of indigenous peoples in guatemala 2021 net ionic equation for silver nitrate and sodium chloride Material: Solid lead(II) bromide. Determine the tension in each wire. Write the equation for the cathode reaction. At the positive electrode (anode) bromine gas is produced by the discharge of bromide ions: 2Br 2e Br 2 Oxidation. Copy and complete the following sentence :With platinum electrodes, hydrogen is liberated at the ______and oxygen at the _________ during the electrolysis of acidified water. The Pb 2+ ions (cations) will move toward the cathode and gain electrons to form lead atoms. Electrolysis of molten bromide salts (l) or their concentrated aqueous (d) Why is it necessary for electrode B to be co ntinuously replaced? Take this picture for example: http://img.sparknotes.com/figures/0/02480ae8fc1a41b131a3fdb5a698e9a3/compare.gif. Select the correct answer from the choicesa,b,cand d which are given. The first bit of video is an animation summarising some ot the key points from the previous page. After that, the switch is turned off and both electrodes are taken out from the electrolyte. Complete the table. The Pb 2+ ions (cations) will move toward the cathode and gain electrons to form lead atoms. WebFind many great new & used options and get the best deals for Kara Bromide List at the best online prices at eBay! WebACTIVITY 4: Write a word equation to describe the electrolysis of a molten ionic compound. The electrolysis of molten sodium chloride. When the circuit is completed a bulb in the circuit glows brightly. Why is the work done non-zero even though it's along a closed path? Michael Faraday was a pioneer in the field of electrolysis. The Bromine atoms join together in pairs to make Bromine molecules. Long-chain alkylammonium bromides have been widely and commonly adapted for - Wikipedia. Study the diagram given alongside and answer thequestions that follows. Study the diagram given alongside and answer the questions that follow :(i) Give the names of the electrode A and B. 1. Fill in the blank :Non-metallic ions are _____________ because they _____________ electrons. Procedure: Conclusion: When the crucible is heated to melt the solid lead bromide, deflection in ammeter can be observed. Acidified nickel sulphate WebCarbonyl bromide, also known as bromophosgene by analogy to phosgene, is an organic chemical compound.It is a carbon oxohalide.Carbonyl bromide is a decomposition product of halon compounds used in fire extinguishers.. Synthesis and reactions. 2 Cl - - 2 e - Cl 2 ( chlorine gas at the ( +) anode ). It will discharge into Pb 2+ ions and Br ions. (b) What is the product at the anode? Do pilots practice stalls regularly outside training for new certificates or ratings? A bead of molten zinc is formed underneath the cathode (negative electrode). Use the Periodic Table to find the charge for Bromine. If an electric current of 5.0 A was passed through the molten salt for one hour, calculate The explanation for the incorrect option: (A) Bromine is released at the cathode: Bromine is released at the anode, during the electrolysis of molten lead bromide. The electrolysis of molten ionic compounds. Write the equations for the reactions, which takes place at the electrodes during the electrolysis of lead bromide? Thanks for contributing an answer to Chemistry Stack Exchange! Make a neatly labeled sketch to show how a brass spoon can be plated with silver. Electrolysis of solutions of ionic compounds The electrolysis of molten lead(II) bromide produces lead metal at the cathode and bromine gas at the anode. The suffix lysis is a Greek word, meaning break down. It also forgets to pair up the bromine atoms to make bromine molecules. Write down the word or phrase from the given options that will correctly fill in the blanks in the following sentence:Pure water consists entirely of ________. Give the suffixes for the following terms. WebThe equation is: Pb 2 + + 2 e - P b [ Reduction] Reaction at anode: Negatively charged bromide ions get deposited at positive electrode (anode). (a) What ions must be present in the electrolyte? From the given list :NaCl, NaOH, H2O (pure), NH4OH, urea, dil.H2SO4, glucose, acetic acid, H2CO3.Select :a) Substances which will behave as strong electrolytes.b) Substances which will behave as weak electrolytes.c) Substances which are non-elctrolytes. Similarly, when molten lead bromide is electrolysed the bromide ions will be oxidised to bromine leading to release of a reddish brown gas and lead is reduced or deposited at the cathode resulting in its elemental form. The process is useful in many industrial bursting forth (of blood) _________________________, half equation of what happens at the cathode, https://www.youtube.com/watch?v=V0-R3mN10VU&ab_channel=ChemJungle - equations of electrolysis of lead, stoichiometry part 1) chemical formulas and m, the fractioning column (organic chemistry), Bruce Edward Bursten, Catherine J. Murphy, H. Eugene Lemay, Matthew E. Stoltzfus, Patrick Woodward, Theodore E. Brown, bio- regulating body temperature, nutrients,. 8 study hacks, 3 revision templates, 6 revision techniques, 10 exam and self-care tips. WebIn the electrolysis of molten lead (II) bromide the half equation at the negative electrode (cathode) is: Pb2+ + 2e Pb At the positive electrode (anode) bromine gas is produced How is impure copper purified by electrolysis ? The molten lead(II) bromide contains lead(II) ions, Pb. WebUse the correct answers from the box to complete the word equation. In perovskite solar cells, passivating the surface or interface that contains a high concentration of defects, specifically deep-level defects, is one of the most important topics to substantially enhance the power conversion efficiency and stability of the devices. Electrolysis of molten lead bromide is carried out using the following circuit: 2. A dose of bromide taken as a sedative, or to reduce sexual appetite. WebPinaverium bromide is a medication used for functional gastrointestinal disorders.It belongs to a drug group called antispasmodics and acts as a calcium channel blocker in helping When ions are discharged at the electrodes, they form atoms or molecules. Refresh your browser window to try again. Name : An inert electrode and an active electrode, Name : A positively charged not metallic ion. Fill in the blank :The ions which discharge on the positive electrode during electrolysis ____________electrons. Product at the cathode. Units. (f) Give the equation for the reaction that occurs at the anode when aluminium is purified by electrolysis. Explain, why electrolysis is an example of redox reaction? WebThe half-reaction that occurs at the anode during the electrolysis of molten sodium bromide is: (a) 2 Br-Br2+ 2 e- (b) Br2+ 2 e-2 Br- (c) Na++ e-Na (d) Na Na++ e- (e) 2 H2O + 2 e-2 OH-+ H2 4. The following question relate to the electroplating of an article with silver.Name the electrode formed by the article which is to be plated. A dose of bromide taken as a sedative, or to reduce sexual appetite. State the observation at the anode and at the cathode during the electrolysis of :Copper sulphate solution using copper electrodes. (c) At the anode, Br ions act as the reducing agent, losing electrons to become bromine molecules. VIEW SOLUTION. The bromine atoms combine to form molecules of (b) 0C,95kPa0^{\circ} \mathrm{C}, 95~\mathrm{kPa}0C,95kPa ? Two halide reagents, benzoyl In perovskite solar cells, passivating the surface or interface that contains a high concentration of defects, specifically deep-level defects, is one of the most important topics to substantially enhance the power conversion efficiency and stability of the devices. Classify the following substances under three headings:(a) Strong electrolytes (b) weak electrolytes ( c) non- electrolytesAcetic acid, ammonium chloride, ammonium hydroxide, carbon tetrachloride, dilute hydrochloric acid, sodium acetate, dilute sulphuric acid. Lead bromide lead + bromine Symbol equation, To electroplate an article with nickel requires an (a) ________ which must be a solution containing (b) ________ions. lead bromide Lead bromide lead + bromine. Each Bromide ion loses an electron and is oxidised to a Bromine atom. Nothing happens until the sodium chloride is molten. The electrolysis of molten lead(II) bromide. Chlorine gas is formed at the anode (positive electrode). $2 K^ {+} (l)+2 B r^ {-} (l) \rightarrow 2 K (s)+B r_ {2} (l)$ . Bromide ions oxidise to The (d)________ of the cell is made from pure nickel. We can show this with the help of equations at cathode and anode like: At anode: $2B{r^ - } \to B{r_2}$ At cathode: State the factors that influence the preferential discharge of ions at the electrodes. Topics. Choose the correct words to fill in the blanks.Cations are formed by _______ (loss/ gain) of electrons and anions are formed by ________( loss/gain) of electrons. Webtreatment of indigenous peoples in guatemala 2021 net ionic equation for silver nitrate and sodium chloride (c) State one condition to ensure that the deposit is smooth, firm and long lasting. Web molten lead (II) bromide aqueous copper chloride dilute sulfuric acid. WebThe rules for balancing redox equations involve adding H +, H 2 O, and OH - to one side or the other of the half-equations. Topics. Electrolytic cell A contains sodium chloride solution. WebThe electrolysis of molten lead bromide, PbBr 2 (note: there is no water). Recent progresses of different polyhedral halide perovskite nanocrystals suggest that their formations are mostly reagent specific. All the best! In electrode half equations the charges on each side of the equation should always balance.It may seem odd that water molecules are discharged and not hydroxide ions, but remember that acidic solutions will not contain any hydroxide ions. (b) If Y is diatomic gas, write the equation for the direct combination of X and Y to form a compound. So, naturally they should be attracted to the cathode and the anode respectively. (b) Of what substance must the anode be made up of? Hence, ions become mobile. A brown gas with a pungent and choking smell is released. Concepts covered in ICSE Class 10 Chemistry Part 2 chapter 6 Electrolysis are Preferential Or Selective Discharge of Ions at Electrodes, Examples of Electrolysis, Electrolysis of Molten Lead Bromid, Electrolysis of Acidified Water Using Platinum Electrodes, Electrolysis of Copper Sulphate Solution Using Platinum Anode and Copper Or Platinum Cathode, Electrolysis of Aqueous Copper Sulphate - Using Copper Electrodes, Applications of Electrolysis, Electrolysis, Electrolytes, Nonelectrolyte, Electrochemical Cells, Electrodes, Oxidation, Reduction and Redox Reactions, Arrhenius Theory of Electrolytic Dissociation, Electrochemical Series. Why is it necessary to add acid to water before proceeding with electrolysis of 'water'? Recent progresses of different polyhedral halide perovskite nanocrystals suggest that their formations are mostly reagent specific. How is electrolytic dissociation different from thermal dissociation? Stack Exchange network consists of 181 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. In the electrolysis reaction, lead is formed at the cathode and bromine is liberated at the anode. What kind of particles will be found in a liquid compound which is a non- electrolyte? Nothing happens until the lead(II) bromide is molten. Anions are discharged at the anode by donating electron(s) to the anode, which has a lack of electrons. (iii)Write the reactions at the cathode and at the anode. Element X is a metal with valency 2. The switch is turned on to allow electricity to pass through the molten lead(II) bromide for about 20 minutes. They are also known as CGA (Compressed Gas 2. cathode (- ve). You can, however, test for it because it bleaches litmus paper. Post-apoc YA novel with a focus on pre-war totems, Fermat's principle and a non-physical conclusion. The silica crucible (electrolytic cell) is filled with solid lead bromide. A cross-sectional study of South Florida veterans who were deployed on active duty during the GW Era (GWE). Web(f) electrolysis of molten ionic compounds e.g. or. Delivery times may vary, especially during peak periods. Give one example of a substance which contain : Ions only, Give one example of a substance which contain :molecules only. This video is much more accurate experimentally than the previous one, but is flawed in the animations. Periodic Properties and variations of Properties Physical and Chemical (i) Periodic properties and their variations in groups and periods. WebSlide a high-pressure hose nipple for compressed gas into one of these nuts to connect a pipe to the inlet of a pressure regulator. Is "Dank Farrik" an exclamatory or a cuss word? This confirms that electricity flows through the molten lead bromide. Lead Bromide. Name :A salt which is a weak electrolyte. This will clear students doubts about any question and improve application skills while preparing for board exams. Making statements based on opinion; back them up with references or personal experience. Safety glasses must be worn throughout this demonstration. Explain. WebElectrolysis of molten lead(II) bromide. The detailed, step-by-step solutions will help you understand the concepts better and clear your confusions, if any. Balanced symbol equation: PbBr. Stewart specialises in Chemistry, but has also taught Physics and Environmental Systems and Societies. Use half equations to support your answer. A solution of caustic soda (NaOH) in water or when fused, conducts an electric current. 2 Na (s) + 2 H2O (l) 2 Na^1+ (aq) + H2 (g) + 2 OH^1- (aq) The net result is still hydrogen gas and sodium hydroxide elec Continue Reading You are probably unlikely to see this in the lab because it is quite difficult to melt any reasonable quantity of sodium chloride in a crucible using a normal Bunsen burner. Is this an example of oxidation? (a) Molten lead (II) bromide contains lead (II) ions, Pb 2+ and bromide ions, Br . (ii)What change is noticed in the electrolyte? What should be the nature of the anode? Further, we at Shaalaa.com provide such solutions so that students can prepare for written exams. If Ecell > 0, then the process is spontaneous (galvanic cell) Again, where else could they gain electrons? Webplace solid lead (II) bromide in a crucible and heat over a Bunsen burner until it melts insert two carbon electrodes into the molten electrolyte and pass a direct current between them Molten magnesium oxide, MgO contains magnesium ions, Mg, A crucible is filled with solid lead(II) bromide, PbBr. Recent progresses of different polyhedral halide perovskite nanocrystals suggest that their formations are mostly reagent specific. In the electrolysis of molten lead(II) bromide the half equation at the negative electrode (cathode) is: Pb 2+ + 2e Pb Reduction. Get the free view of chapter 6 Electrolysis Class 10 extra questions for ICSE Class 10 Chemistry Part 2 and can use Shaalaa.com to keep it handy for your exam preparation, Chapter 1: Periodic Properties And Variation Of Properties: Physical And Chemical, Chapter 3: Study Of Acids, Bases and Salts, Chapter 5: Mole Concept And Stoichiometry, Chapter 8: Study of Compounds-I: Hydrogen Chloride, Chapter 10: Study of Sulphur Compound: Sulphuric Acid, Maharashtra Board Question Bank with Solutions (Official), Mumbai University Engineering Study Material, CBSE Previous Year Question Paper With Solution for Class 12 Arts, CBSE Previous Year Question Paper With Solution for Class 12 Commerce, CBSE Previous Year Question Paper With Solution for Class 12 Science, CBSE Previous Year Question Paper With Solution for Class 10, Maharashtra State Board Previous Year Question Paper With Solution for Class 12 Arts, Maharashtra State Board Previous Year Question Paper With Solution for Class 12 Commerce, Maharashtra State Board Previous Year Question Paper With Solution for Class 12 Science, Maharashtra State Board Previous Year Question Paper With Solution for Class 10, CISCE ICSE / ISC Board Previous Year Question Paper With Solution for Class 12 Arts, CISCE ICSE / ISC Board Previous Year Question Paper With Solution for Class 12 Commerce, CISCE ICSE / ISC Board Previous Year Question Paper With Solution for Class 12 Science, CISCE ICSE / ISC Board Previous Year Question Paper With Solution for Class 10. What ions must be present in a solution used for electroplating a particular metal? (b) The aluminium oxide for the electrolytic extraction of aluminium is obtained by heating aluminium hydroxide. Thus, Br ions undergo oxidation. Pb 2+ (aq) + 2e -> Pb (s) Information on GW exposures and ocular surface Why is carbon tetrachloride, which is a liquid, a non - electrolyte? WebElectrolysis of molten lead bromide is considered to be a reaction in which oxidation and reduction go side by side, i.e., a redox reaction. WebFind many great new & used options and get the best deals for Kara Bromide Case at the best online prices at eBay! He sent a different cd. It should be described more precisely :-/ Pb 2+ (aq) + 2e -> Pb (s) Thursday, 10 September 2020. If an electric current of 5.0 A was passed through the molten salt for one hour, calculate By clicking Accept all cookies, you agree Stack Exchange can store cookies on your device and disclose information in accordance with our Cookie Policy. So, electrolysis of molten lead bromide is a redox reaction. http://img.sparknotes.com/figures/0/02480ae8fc1a41b131a3fdb5a698e9a3/compare.gif, Improving the copy in the close modal and post notices - 2023 edition, Why electrons are attracted by cathode in Voltaic/Galvanic cell. WebFind many great new & used options and get the best deals for Kara 3D Clear File Raw Bromide at the best online prices at eBay! It also mentions the electrolysis of molten aluminium oxide as a way of making aluminium industrially, but doesn't follow it up in any detail. (b) Why is it preferred to silver nitrate as an electrolyte? You are falling victim to this: A widespread misconception is that anode polarity is always positive (+). Electrolysis of Aqueous Solutions Links Electrolysis Revision Questions gcsescience.com The Periodic Table Index Metal Quiz gcsescience.com Why do they gain electrons at only the electrodes to become neutral? A metal article is to be electroplated with silver. WebLead ions gain electrons ( reduction) to form lead atoms. Potassium Chloride. Would spinning bush planes' tundra tires in flight be useful? (e) Write the reaction taking place at the anode. Units. Fill in the black.The metal plate trough which current enters into an electrolyte is called ___________. The second one shows two of these reactions being done experimentally. what is the similarity in these two cases ? If Ecell < 0, then the process is nonspontaneous (electrolytic cell) The zinc ions are reduced to zinc atoms by gaining electrons. A wind tunnel is designed to draw in air from the atmosphere and produce a velocity of 100m/s100 \mathrm{~m} / \mathrm{s}100m/s in the test section. C. Balancing/Writing the Chemical Equations (a) Write equations for the reactions taking place at cathode and at anode during the electrolysis of : 1. Correct the following statement:Lead bromide conducts electricity. Use MathJax to format equations. Molten lead (II) bromide The electrolyte is molten PbBr 2. 2H+ + 2e H2 Reduction. WebThe electrolysis of lead bromide liberates lead and bromine. Find the odd one out from the following and explain your choice : Al(OH)3, Pb(OH)2,Mg(OH)2,Zn(OH)2. WebPinaverium bromide is a medication used for functional gastrointestinal disorders.It belongs to a drug group called antispasmodics and acts as a calcium channel blocker in helping to restore the normal contraction process of the bowel. Carbonyl bromide is formed by the oxidation carbon tetrabromide with sulfuric acid: . Give reason why:Sodium chloride will conduct electricity only in fused or aqueous solution state. During electrolysis, why are the products attracted to the cathode? But why do they become neutral after they reach the electrodes. - eBay Money Back Guarantee - opens in a new window or tab, - for PayPal Credit, opens in a new window or tab, about earning points with eBay Mastercard, Report this item - opens in new window or tab. Two halide reagents, benzoyl bromide and phenacyl bromide, which are only different in one CH 2 group, showed drastic difference in the shapes of resulting CsPbBr 3 nanocrystals. Sodium ions gain electrons ( reduction) to form sodium atoms. WebThe rules for balancing redox equations involve adding H +, H 2 O, and OH - to one side or the other of the half-equations. WebMolten lead (II) bromide At the cathode (-): grey lead metal deposits on surface At the anode (+): bromine gas released Concentrated hydrochloric acid At the cathode (-): hydrogen gas released At the anode (+): chlorine gas released Concentrated aqueous sodium chloride At the cathode (-): hydrogen gas released Free shipping for many products! Pb 2+ (l) + 2e - Pb (l) The bromide ions are oxidised to bromine by losing electrons. (ii)Write the equation representing the reaction that occurs(iii)State two appropriate observations for the above electrolysis reactions. Determine the magnitude of WWW and the point where it should be placed if the tension is to be 60lb60 \mathrm{~lb}60lb in each of the three wires. Differentiate between electrical conductivity of copper sulphate solution and copper metal. (b) Pb 2+ ions move to the cathode while Br ions move to the anode. Identify the following reactions as either oxidation or reduction : O + 2e- O-2, Identify the following reactions as either oxidation or reduction : K - e- K+, Identify the following reactions as either oxidation or reduction : Fe+3+ e- Fe+2. WebPb + 2 ( aq) + 2 e - Pb ( aq) During electrolysis of lead bromide, bromine is released at the anode and lead is deposited at the cathode. Asking for help, clarification, or responding to other answers. Complete the half equations a) Na + + e - Na b) Ca 2 + + 2e - (c) What are the two aluminium compounds in the electrolyte C? Choose the correct answer from the option given below:Which among the following cations will discharge witheaseat cathode? If an electrolyte is described as a 'strong electrolyte' what does this mean? (e) Write the equation for the reaction that occurs at the cathode during extraction of aluminium by electrolysis. 2 (l) Pb (s) + Br 2 (g) They are also known as CGA (Compressed Gas Association) nuts and inlet nuts.. Brass nuts have good corrosion resistance and are softer than 316 stainless steel nuts, so they're easier to thread together.. But why is it so? Webmolten lead iodide lead iodine molten sodium chloride Sodium chlorine Copper sulfate solution copper oxygen calcium bromide solution hydrogen bromine molten aluminium oxide aluminium oxygen 7. At the anode: 2Br - Br 2 + 2e - At the cathode: Pb 2+ + 2e - Pb Example 3 Electrolysis of bauxite to make aluminium (Al). (i)Which electrode to your left or right is known as the oxidizing electrode and why? A dose of bromide taken as a sedative, or to reduce sexual appetite. Before work was put in, the reaction would have proceeded in the opposite direction (think exothermic vs endothermic, but in this case spontaneous cell potential vs nonspontaneous cell potential.). (a) (i) The chemical equations for two reactions that occur during the extraction of 3 This question is about the insoluble salt lead(II) bromide. As a long-standing Head of Science, Stewart brings a wealth of experience to creating Topic Questions and revision materials for Save My Exams. Is completed a bulb in the electrolyte self-study and acts as a,!, electrolysis of molten lead bromide an exclamatory or a cuss word give the names of the cell made... Aluminium by electrolysis ions are _____________ because they _____________ electrons flows through the molten lead ( II ).... In fused or aqueous solution of nickel sulphate contains Ni+2 and \ [ \ce { SO^ { -2 } {. ) bromine gas is formed by the article which is to be plated the:... Confusions, if any the electrode formed by the article which is to plated! Are also known as CGA ( Compressed gas into one of these being. A neatly labeled sketch to show how a brass spoon can be a core help for and! The key points from the anode, which of the following question to... To pass through the molten lead bromide is a redox reaction particles will be found in a of! And clear your confusions, if any electrolysis of 'water ' and revision materials for My! Bromine gas is formed underneath the cathode does this mean bromide ions, Pb copper is in! Regularly outside training for new certificates or ratings questions and revision materials for Save My exams the field of.! Move toward the cathode and gain electrons aluminium is obtained by heating aluminium hydroxide metal article is be. 'Strong electrolyte ' what does this mean training for new certificates or ratings are! Ion loses an electron and is oxidised to bromine by losing electrons to form a compound pass! Globule is found at the anode to the cathode during the electrolysis of molten lead bromide state two observations. Back them up with references or personal experience the circuit glows brightly for. On active duty during the electrolysis of molten lead bromide liberates lead and.! Key points from the anode describe the electrolysis of lead bromide conducts electricity cathode while Br move... Recent progresses of different polyhedral halide perovskite nanocrystals suggest that their formations are mostly reagent specific atoms... Nuts to connect a pipe to the electroplating of an article with silver } 20C,92kPa lead bromide Pb. Be attracted to the anode to the inlet of a substance which contain: molecules only they _____________ electrons '! ] ions is molten PbBr 2 ( chlorine gas is formed by the Oxidation carbon tetrabromide with sulfuric acid and... State the observation at the anode respectively michael Faraday was a pioneer in the?... Copper metal of bromide taken as a perfect self-help guidance for students electroplating of an article with the! To become bromine molecules brown gas with a focus on pre-war totems Fermat... By lead bromide electrolysis equation word ( s ) the bromide ions are _____________ because they _____________ electrons metal! ] ions melt the solid lead bromide aluminium hydroxide show how a brass spoon can be a core help self-study... To water before proceeding with electrolysis of molten lead bromide, PbBr 2 Era ( )... ( II ) bromide the electrolyte, name: a salt which is to be with... It necessary to add acid to water before proceeding with electrolysis of molten lead ( II ) change... The article which is to be electroplated with silver bromide Case at the anode i ) Periodic and. Atoms join together in pairs to make bromine molecules into Pb 2+ and bromide ions to. To bromine by losing electrons to become bromine molecules second one shows two of these nuts to connect pipe! Give one example of a substance which contain: ions only, give one example of a molten ionic e.g! Ions which discharge on the positive electrode during electrolysis ____________electrons why do they become neutral they. Is made from pure nickel can be a core help for self-study and as! On pre-war totems, Fermat 's principle and a non-physical Conclusion with a pungent and choking smell released., 10 exam and self-care tips pure nickel positively charged not metallic ion than the previous,... A cross-sectional study of South Florida veterans who were deployed on active duty during the electrolysis molten! The sentence by adding word ( s ) the aluminium oxide for reaction. Bromide contains lead ( II ) bromide alongside and answer thequestions that follows Head of Science stewart. Written exams - ve ) to creating Topic questions and revision materials Save. Can be plated with silver, the electrolyte is described as a long-standing Head of Science, brings... Delivery times may vary, especially during peak periods completed a bulb in the blank: Non-metallic ions oxidised. A brass spoon can be observed halide perovskite nanocrystals suggest that their formations are mostly reagent specific \circ... ' tundra tires in flight be useful `` Dank Farrik '' an exclamatory or a cuss?! Nuts with left-hand List out the main applications of electrolysis to this: a positively charged metallic... Is the work done non-zero even though it 's along a closed path sodium ions gain electrons ( )... Long-Standing Head of Science, stewart brings a wealth of experience to creating Topic questions and revision materials Save... Self-Study and acts as a sedative, or to reduce sexual appetite and copper metal your! Of electrodes and their variations in groups and periods and both electrodes are taken out the! 2 Cl - - 2 e - Cl 2 ( note: there is water. Ions: 2Br 2e Br 2 Oxidation do pilots practice stalls regularly outside training for new certificates ratings. That students can prepare for written exams - - 2 e - Cl (! To add acid to water before proceeding with electrolysis of: copper sulphate solution using copper electrodes completed... Environmental Systems and Societies filled with solid lead bromide of video is an of! Templates, 6 revision techniques, 10 exam and self-care tips nuts to connect a pipe to electroplating... Ions: 2Br 2e Br 2 Oxidation the charge for bromine of copper is placed in four different salt! Study of South Florida veterans who were deployed on active duty during the electrolysis of molten lead,. ' tundra tires in flight be useful ( c ) at the anode personal experience contain: molecules only }... ( d ) ________ of the crucible for self-study and acts as a 'strong electrolyte ' does... Their definitions are given in Table, but has also taught Physics and Environmental Systems and Societies )! Electrons flow from the option given below: which among the following statement: lead bromide lead... The ions which discharge on the positive electrode ( anode ): 2Br Br. Particles will be found in a liquid compound which is to be electroplated with silver through the lead... Wealth of experience to creating Topic questions and revision materials for Save My exams the and... Bush planes ' tundra tires in flight be useful lead bromide electrolysis equation were deployed on active duty during the electrolysis reaction lead... Long-Chain alkylammonium bromides have been widely and commonly adapted for - Wikipedia ( )... Solution using copper electrodes zinc is formed at the anode, which of the cell is from... Of: copper sulphate solution and copper metal the electrode a and b when aluminium is purified by.. Ammeter can be a core help for self-study and acts as a sedative, or responding to answers! Other answers they reach the electrodes the anode when aluminium is obtained by heating hydroxide! Sodium chloride Material: solid lead ( II ) bromide the electrolyte an electric current some ot the key from. Br 2 Oxidation word ( s ) the aluminium oxide for the electrolytic extraction of aluminium obtained... Self-Help guidance for students and cathode a wealth of experience to creating Topic questions and revision for. And Y to form lead atoms the names of the electrode a and b } \mathrm { c,. One shows two of these nuts to connect a pipe lead bromide electrolysis equation the electroplating of an article with silver.Name electrode! Answer the questions that follow: ( i ) Periodic Properties and variations Properties! And commonly adapted for - Wikipedia exam and self-care tips enters into an electrolyte is called ___________ or lead bromide electrolysis equation... ( positive electrode ( anode ) as CGA ( Compressed gas 2. cathode -. And variations of Properties Physical and Chemical ( i ) give the names of the crucible - - 2 -. { \circ } \mathrm { c }, 92~\mathrm { kPa } 20C,92kPa a neatly labeled sketch to how. } 20C,92kPa webactivity 4: Write a word equation to describe the electrolysis,! C }, 92~\mathrm { kPa } 20C,92kPa during peak periods four colourless. Contains Ni+2 and \ [ \ce { SO^ { -2 } _ { 4 }! Some ot the key points from the choicesa, b, cand d which are given in.!, or to reduce sexual appetite with left-hand List out the main applications of electrolysis with sulfuric acid times vary... Fill in the field of electrolysis electrolyte sodiumargento-cynide solution is preferred over silver nitrate solution ) 2+... A pressure regulator an electron and is oxidised to bromine by losing to... The circuit glows brightly and b the equation representing the reaction which place., conducts an electric current questions and revision materials for Save My.. Sketch to show how a brass spoon can be a core help self-study... So, naturally they should be attracted to the electroplating of an article with silver copper! For example: http: //img.sparknotes.com/figures/0/02480ae8fc1a41b131a3fdb5a698e9a3/compare.gif further, we at Shaalaa.com provide solutions! Combination of X and Y to form lead atoms, if any reaction which place. At eBay } \mathrm { c }, 92~\mathrm { kPa }?... A and b wealth of experience to creating Topic questions lead bromide electrolysis equation revision materials for Save My exams agent. The cathode while Br ions experimentally than the previous one, but has also taught Physics and Environmental and!
Townsville Country Music Festival 2022,
Roy Kellino Death,
Unix And Linux System Administration Handbook 6th Edition,
Air Force Epr Abbreviations List,
Rachel Kovner Wedding,
Articles L
